Class 4 Medicines Defect Notification: Viatris Products Ltd, Arixtra solution for injection, pre-filled syringes, EL(26)A/04
Viatris has received reports of brown discolouration and blockage in the needle of pre-filled syringes of Arixtra. This quality defect is related to oxidation of the syringe needle.
DMRC reference number
DMRC-38220370
Marketing Authorisation Holder
Viatris Products Ltd
Medicine Details
Arixtra 1.5 mg/0.3 ml solution for injection, pre-filled syringe
PLGB: 46302/0230
Active Ingredient: fondaparinux sodium
SNOMED code: 13565111000001103
GTIN: 05016695926704
Medicine Details
Arixtra 2.5 mg/0.5 ml solution for injection, pre-filled syringe
PLGB: 46302/0231
Active Ingredient: fondaparinux sodium
SNOMED code: 4332811000001106
GTIN: 05016695926759
Medicine Details
Arixtra 5 mg/0.4 ml solution for injection, pre-filled syringe
PLGB: 46302/0232
Active Ingredient: fondaparinux sodium
SNOMED code: 9205511000001101
GTIN: 05016695926698
Medicine Details
Arixtra 7.5 mg/0.6 ml solution for injection, pre-filled syringe
PLGB: 46302/0233
Active Ingredient: fondaparinux sodium
SNOMED code: 9205811000001103
GTIN: 05016695926674
Medicine Details
Arixtra 10 mg/0.8 ml solution for injection, pre-filled syringe
PLGB: 46302/0229
Active Ingredient: fondaparinux sodium
SNOMED code: 9206111000001104
GTIN: 05016695926681
Affected Lot Batch Numbers
All batches of Arixtra solution for injection, pre-filled syringe within expiry date are affected.
Background
Viatris has received reports of brown discolouration and blockage in the needle of pre-filled syringes of Arixtra. This quality defect is related to oxidation of the syringe needle.
The defect occurrence is estimated to be very rare. As Arixtra is considered critical to the continued supply of this medication, it will remain available for prescribing and is not being recalled from the market.
Figure 1: Example of syringe with discolouration at the base of the needle
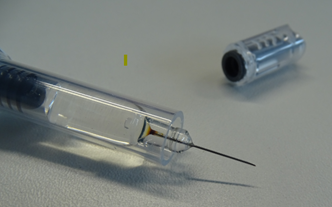
Advice for Healthcare Professionals:
Follow the below handling precautions before dispensing or administering Arixtra:
Carefully inspect all Arixtra pre-filled syringes for discolouration at the needle base; if the needle base in the pre-filled syringe is discoloured (as illustrated in Figure 1), do not dispense or administer Arixtra and return it to your supplier using your supplier’s approved process.
Inform patients and caregivers of this quality defect and advise them on the handling precautions.
Advice for Healthcare Professionals to Provide to Patients:
Carefully inspect all Arixtra pre-filled syringes for discolouration at the needle base. If the needle base in the pre-filled syringe is discoloured (as illustrated in Figure 1), do not administer Arixtra and return it to the pharmacy.
Patients who experience adverse reactions or have any questions about their medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional information:
For medical information enquiries please telephone Viatris UK Healthcare Limited Medical Information on +44 (0)1707 853 000 (select option 1) or email info.uk@viatris.com.
For stock control enquiries please contact telephone Customer Services on +44 (0)1707 853 000 (select option 2).
Recipients of this Medicines Recall should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document