Class 3 Medicines Recall: Activase Pharmaceuticals Limited, Prednisolone 5mg Soluble Tablets, EL(25)A/54
Activase Pharmaceuticals Limited is recalling two batches of Prednisolone 5mg Soluble Tablets as a precautionary measure due to a limited number of reports of blister pockets becoming swollen over time.
DMRC reference number
DMRC-37820855
Marketing Authorisation Holder
Activase Pharmaceuticals Limited
Medicine Details
Prednisolone 5mg Soluble Tablets
PL: 28444/0276
Active ingredient: prednisolone sodium phosphate
SNOMED code: 44943411000001106
GTIN: 05060014446695
Affected Lot Batch Numbers
| Batch No. | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| 2409006 | 31/05/2027 | 30 | 06/03/2025 |
| 2409007 | 31/05/2027 | 30 | 06/05/2025 |
Background
Activase Pharmaceuticals Limited is recalling two batches of Prednisolone 5mg Soluble Tablets as a precautionary measure due to a limited number of reports of blister pockets becoming swollen over time.
The blister pocket swelling is due to gas (carbon dioxide) build up, from gradual excipient reaction with trace amounts of moisture. The image below shows an impacted blister strip.
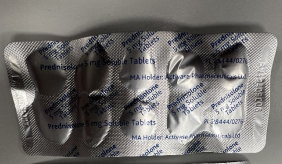
Advice for Healthcare Professionals:
Stop supplying the above batches immediately. Quarantine any remaining stock and return it to your supplier, using your supplier’s approved process.
Activase Pharmaceuticals Limited have confirmed that this issue is limited to the batches listed in this notification and that no other batches are affected.
Advice for Patients:
Patients should continue to take the medication from these batches unless the blister strips exhibit signs of swelling as the quality of the tablets will not be impacted. If you have received one of the batches of medication and the blister strip exhibits swelling, return it to your dispensing pharmacy. If you have any concerns about your medication speak to your pharmacy in the first instance.
This recall is being actioned at pharmacy and wholesaler level as a precautionary measure.
Patients who experience adverse reactions or have any questions about their medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional information:
For all medical information enquiries and information on this product, please email eupvg@genreg.eu or telephone 020 72010421.
For stock control enquiries please email info@genesis-pharma.com or telephone 0207 2010400.
Recipients of this Medicines Recall should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document