Class 2 Medicines Recall: Flutiform 250 micrograms / 10 micrograms per actuation pressurised inhalation, suspension , CD Pharma Ltd, EL(25)A/35
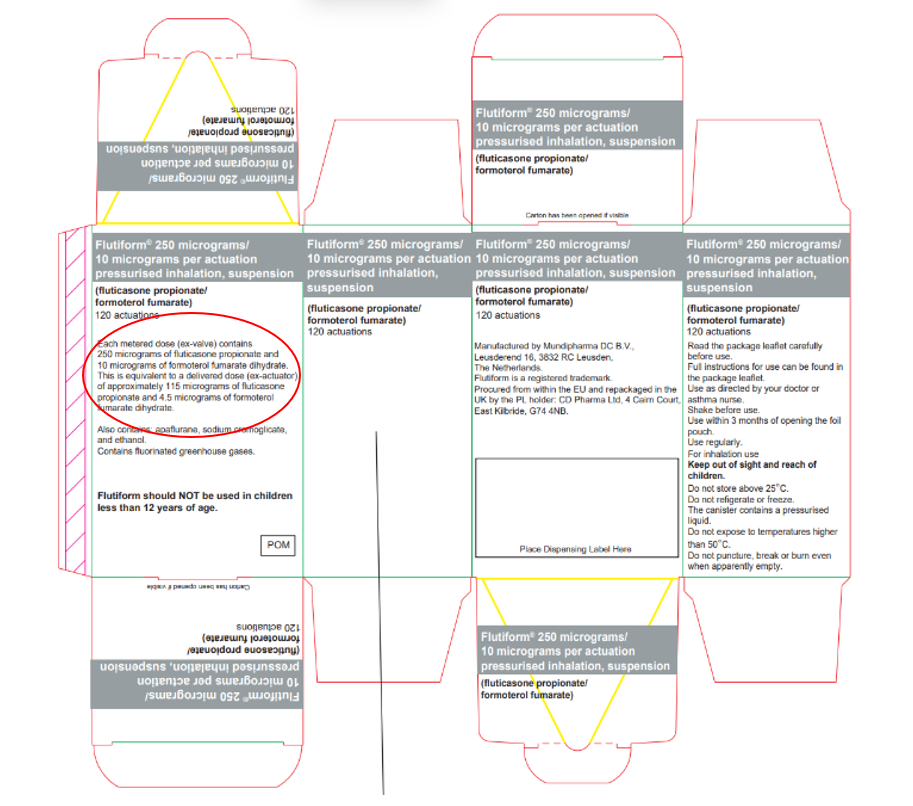
CD Pharma Ltd have notified the MHRA of an error on the outer carton of the product for the batches listed in this notification. While the total active content statement is correct, the delivered dose content statement is incorrect. The other details on the carton are correct.
DMRC reference number
DMRC-36216704
Marketing Authorisation Holder
CD Pharma Ltd
Medicine Details
Flutiform 250 micrograms / 10 micrograms per actuation pressurised inhalation, suspension
PL: 20492/0724
Active Ingredient: fluticasone propionate/formoterol fumarate
SNOMED code: n/a
GTIN: 05060011835409
Affected Lot Batch Numbers:
| Batch No. | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| 270185-24062FA-30424 | 30 Jun 2026 | 120 Actuations | 30 Jun 2025 |
| 270186-24065FA-30443 | 30 Jun 2026 | 120 Actuations | 04 Jul 2025 |
| 270774-24082FB-30444 | 31 Jul 2026 | 120 Actuations | 04 Jul 2025 |
| 269881-24064FA-30425 | 30 Jun 2026 | 120 Actuations | 04 Jul 2025 |
Background:
CD Pharma Ltd have notified the MHRA of an error on the outer carton of the product for the batches listed in this notification. While the total active content statement is correct, the delivered dose content statement is incorrect.
| Incorrect active content statement | Correct active content statement |
|---|---|
| ‘Each metered dose (ex-valve) contains 250 micrograms of fluticasone propionate and 10 micrograms of formoterol fumarate dihydrate. This is equivalent to a delivered dose (ex-actuator) of approximately 115 micrograms of fluticasone propionate and 4.5 micrograms of formoterol fumarate dihydrate.’4 | ‘Each metered dose (ex-valve) contains 250 micrograms of fluticasone propionate and 10 micrograms of formoterol fumarate dihydrate. This is equivalent to a delivered dose (ex-actuator) of approximately 230 micrograms of fluticasone propionate and 9 micrograms of formoterol fumarate dihydrate.’ |
The other product details on the carton, including the name, strength and pharmaceutical form of the medicine are correct. The quality of the medicine is not impacted by the labelling defect.
Advice for Healthcare Professionals:
Stop supplying the above batch immediately. Quarantine all stock and return it to your supplier using your supplier’s approved process.
Advice for Patients:
Patients who are taking Flutiform 250 micrograms / 10 micrograms per actuation pressurised inhalation, suspension should continue to take the medication as prescribed by their healthcare professional. This issue only impacts the statement related to the delivered dose and the other product details on the carton, including the name, strength and pharmaceutical form of the medicine are correct.
This is a wholesale and pharmacy level recall that will be actioned by a healthcare professional. There is no quality issue with the product and patients can continue to take their medicine as prescribed.
Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional information:
For all further enquiries please contact 01355 204 448 or email regaffairs@teleta.co.uk
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document