Class 2 Medicines Recall: Depo-Medrone 80 mg in 2 mL, Maxearn Limited EL(25)A/29
A batch of Depo-Medrone has been released to the market with an error. The vial over label incorrectly states that the total vial content is 40 mg in 1 mL, when the correct total vial content is 80mg in 2 mL (with a concentration of 40mg/ml of methylprednisolone acetate).
DMRC reference number
DMRC-35933447
Marketing Authorisation Holder
Quadrant Pharmaceuticals Limited / Maxearn Limited
Medicine Details
Depo-Medrone 80mg/2mL suspension for injection
PLPI 20774/1666
Active ingredient: methylprednisolone acetate
SNOMED code: N/A
GTIN: 5060537815503
Affected Lot Batch Numbers:
| Batch No. | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| LG3883 Max 0943Y | 29/02/2029 | 2mL | 13/05/2025 |
Background:
Maxearn Limited have informed the MHRA that the label on an imported batch of Depo-Medrone has been released to the market with an error. The vial label contains incorrect information regarding volume and total vial content. The label incorrectly states that the total vial content is 40 mg in 1 mL, when the correct total vial content is 80mg in 2 mL (with a concentration of 40mg/ml of methylprednisolone acetate). The error could result in the administration of the incorrect dose.
Maxearn has contacted their distributor, 32 packs have been distributed to pharmacies. The remaining units provided to the distributor have been quarantined.
The error only impacts the parallel imported product repackaged by Maxearn, packs from other sources are not affected and should continue to be dispensed.
Image of incorrect over label and impacted vial:
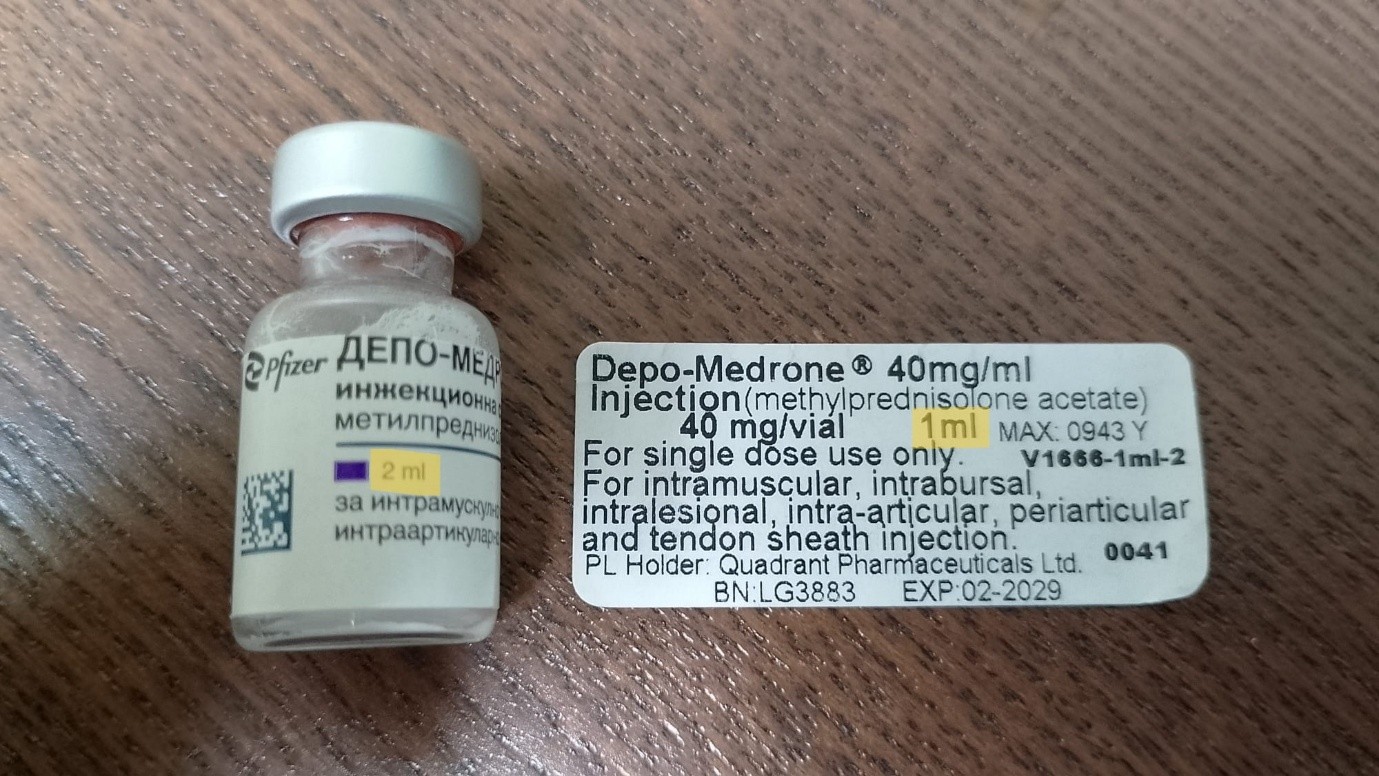
image of correct over label:

Advice for Healthcare Professionals:
Stop supplying the above batch immediately. Quarantine all stock and return it to your supplier using your supplier’s approved process.
Maxearn can confirm 32 packs were released by the distributor. All other units have been held before onward distribution. Maxearn will arrange for customers who received these units to be contacted.
Healthcare professionals who have been supplied this batch and have administered the medicine to patients should contact the patient to make them aware of this error and provide any relevant clinical advice.
Advice for Patients:
No further action is required by patients as this is a Pharmacy and Wholesaler level recall. Depo-Medrone is administered under the supervision of a healthcare professional. Patients who have received this batch will be contacted by your healthcare professional.
Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional information:
For all medical information enquiries and information on this product, please email maxearnqa@maxearn.co.uk or telephone 01204 471 269.
For stock control enquiries please email maxearnqa@maxearn.co.uk or telephone 01204 471 269.
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document