Genetic Modification Inspectorate: seed audit programme
Guidance on the Genetic Modification Inspectorate (GMI) seed audit programme for companies in England that import, produce or market seed.
Applies to England
Genetically modified (GM) seed audits are carried out by, or on behalf of, UK authorities in:
- England – the Department for Environment, Food and Rural Affairs (Defra)
- Northern Ireland – the Department of Agriculture, Environment and Rural Affairs (DAERA)
- Scotland – the Scottish Government – contact the SASA GMI
- Wales – the Welsh Government
Seed audit programme in England
The programme helps companies in England comply with the rules on genetically modified organisms (GMOs) in seeds by providing evidence of the actions they take.
The audits include seeds that are to be:
- marketed for commercial production
- used for trial purposes
The audits are carried out by the Animal and Plant Health Agency (APHA) Genetic Modification Inspectorate (GMI) on behalf of Defra.
Taking part in audits is voluntary but you must always follow the relevant legislation and must not import or produce seeds that contain unauthorised GMOs. For more information, read Part VI, Section 8, sub-section (2) of the Environmental Protection Act (1990).
This guide is updated annually before the next round of audits programme begins.
Seed company responsibilities
Companies that import or produce conventional seed in the UK must get consent before they import, acquire, release or market genetically modified organisms (GMOs). Conventional seed means seed produced to relevant certification standards, that is destined for sowing and has not been genetically modified (GM).
They must put appropriate controls in place to minimise the risk of adventitious GM presence (AGMP) (the unexpected, accidental or unplanned occurrence of GM seeds) in the seed for sowing.
Importing or producing seed
You need to show the auditor you have taken action to minimise the risk of AGMP in seed you market or enter into trials.
Crops most at risk of GM presence
The following crop species have been assessed by the GMI as being most at risk of AGMP:
- Brassica napus: winter and spring oilseed rape, swede, swede fodder rape, salad rape, rape or kale hybrids
- Brassica rapa: turnip, turnip fodder rape, stubble turnips, Chinese cabbage, pak choi, oriental greens, rapid-cycling brassicas
- Glycine max: soya bean, edamame
- Zea mays: fodder maize, grain maize and sweetcorn
Control measures to protect seed purity
The main approaches that companies use to protect the purity of their seed in terms of GMOs are production controls and analytical tests.
1. Production controls include:
These include, but are not limited to:
- seed provenance – documentation detailing the source of the germplasm, including the breeder and country of origin, and confirmation that the seed has been produced from non-GM lines
- variety maintenance – evidence that the variety has been maintained in isolation from transgenic lines (for example by the use of spatial, temporal, physical or procedural methods)
- seed production controls – documentation detailing the controls in place to prevent contamination in the field, including at sowing, during the growing or flowering stage, and at harvest
- controls relating to transport, storage and processing – documentation confirming the use of suitable measures to prevent the introduction of AGMP due to admixture with other seed (contracted processors should provide written assurances to demonstrate they have appropriate measures in place to minimise the risk of seed acquiring AGMP)
If suppliers request written statements (letters of assurance) as a proxy for direct control or knowledge of production controls, the statements should refer to the specific relevant controls and how they have been applied to the seed.
2. Analytical tests
Analytical tests on individual seed batches or lots, include:
- DNA-based tests, for example, polymerase chain reaction (PCR) tests
- protein-based tests, for example, lateral flow devices (LFDs) or lateral flow tests (LFTs)
- spray tests, for example, for herbicide tolerance
As a minimum, each analytical test should:
- be carried out on a representative sample of the seed lot
- be carried out on a minimum working sample for analysis that contains at least 3000 seeds (which may need to be tested in batches to align with analytical sensitivity)
- include appropriate positive and negative controls
- address the risk of possible false positives
- be conducted to an analytical sensitivity level of at least 0.1% (for example, a limit of detection of less than or equal to 0.1%)
- indicate any standards or accreditation it conforms to, for example ISO17025, United Kingdom Accreditation Service (UKAS), Association Française de Normalisation (AFNOR), Good Laboratory Practice (GLP)
Each analytical test should include the result for the test with the associated measurement of uncertainty for the result (for example, plus or minus 95% confidence limits).
PCR-based tests
Consult with an accredited laboratory about the sequences that can be targeted. This should include:
- a combination of common promotor and terminator sequences
- antibiotic markers appropriate to the crop in question
- testing for sequences that encode for specific traits
Due to the prevalence of cauliflower mosaic virus in brassica crops, it is not recommended to use p35S as a target when testing brassica seed for GM presence. This is because cauliflower mosaic virus is the natural source of the common GM promoter p35S. You should contact the Genetic Modification Inspectorate to discuss PCR-based testing.
Assurances should be provided on the separate handling of the seed subsequent to any testing. Test certificates should clearly identify the lots or batches they refer to.
Mutagenesis and gene-editing
In April 2022, the law changed regarding genetically modified plants under the Genetically Modified Organisms (Deliberate Release) (Amendment) (England) Regulations 2022). These changes affect field trials for research and development only and not commercialisation or marketing. These plants are described as ‘qualifying higher plants’, material from which must not be allowed to enter the human food chain or fed to animals.
The Genetic Modification Inspectorate carries out inspections of Genetically Modified Organism field trials in accordance with the powers granted under the Environmental Protection Act 1990, including for qualifying higher plants where there is a possibility of non-compliance.
On 25 July 2018, the European Court of Justice (case C-528/16) ruled that the use of certain gene editing techniques, such as oligonucleotide-directed mutagenesis (including CRISPR-Cas9 techniques), constitute genetic modification. This means that, within the EU, all organisms produced by gene-editing techniques must be regulated as GMOs.
Plant varieties produced in the EU using these techniques need to go through the EU authorisation process before being marketed or commercially cultivated in the EU and Northern Ireland.
In Great Britain, separate authorisations are required for the import, acquisition, release or marketing of GMOs in England, Scotland and Wales.
Before the ruling some breeders were known to be preparing to market varieties produced using these now-regulated techniques. The GMI does not believe that any such products have been marketed in the EU. However, companies should remain vigilant to the potential use of regulated gene-editing techniques. If you have any concerns regarding the provenance of varieties you are intending to market, contact the GMI.
Genetic Technology (Precision Breeding) Regulations
The Genetic Technology (Precision Breeding) Regulations 2025 (‘the regulations’) passed into law 13 May 2025 and came into effect 13 November 2025. The regulations provide the operational implementation of the Genetic Technology (Precision Breeding) Act 2023 (‘the act’) and both apply to England only.
The regulations outline the process for the release and marketing of precision bred plants and food and feed derived from them. A plant is precision bred if all features in its genome produced using modern biotechnology could have occurred through traditional processes.
Following the act’s introduction, further regulatory amendments and legislation are needed to allow variety listing and marketing of precision bred seed and plant reproductive material. Once introduced, varieties can be examined for listing and certification as precision bred organisms rather than GMOs, facilitating the marketing of these new plant varieties.
GM presence in Zea mays
If a PCR test on an individual batch or seed lot shows AGMP at any detection level, you cannot market or plant any of the seeds (except Zea mays). Contact the GMI for advice.
For Zea mays there is an authorisation for commercial cultivation of GM maize in England, for MON810 (notification C/F/95/12/02, event code MON-ØØ81Ø-6, Monsanto).
There is no marketing restriction on approved GMOs which have been cleared as presenting no risk to human health or to the environment. However, you must label seeds, as described in Regulation 1830/2003 (as amended).
In the absence of specific labelling thresholds in English legislation for the adventitious presence of approved GMOs in conventional seed, UK industry has chosen to adopt a precautionary approach and operates to the level of detection (0.1% or less). If you market seed in England with a detectable level of an approved GMO, you must declare that level on the seed label. You cannot market seed with any level of an unapproved GMO.
PCR junction primer tests
A number of GM elements are derived from naturally occurring bacteria and viruses. PCR tests targeting these elements can give false positive results, as the presence of such bacteria and viruses in seed can give a positive result.
PCR junction primer tests target the adjoining regions between neighbouring GM elements that would not normally be associated in nature. This makes these tests less likely to produce false positive results compared to standard PCR tests. However, because junction-spanning tests have an increased specificity, they target a smaller range of elements, resulting in a potential reduction in the number of GM lines the test can detect.
This is illustrated in Figure 1, which shows a test targeting the p35S and NPTII junction of a GM construct.
Figure 1
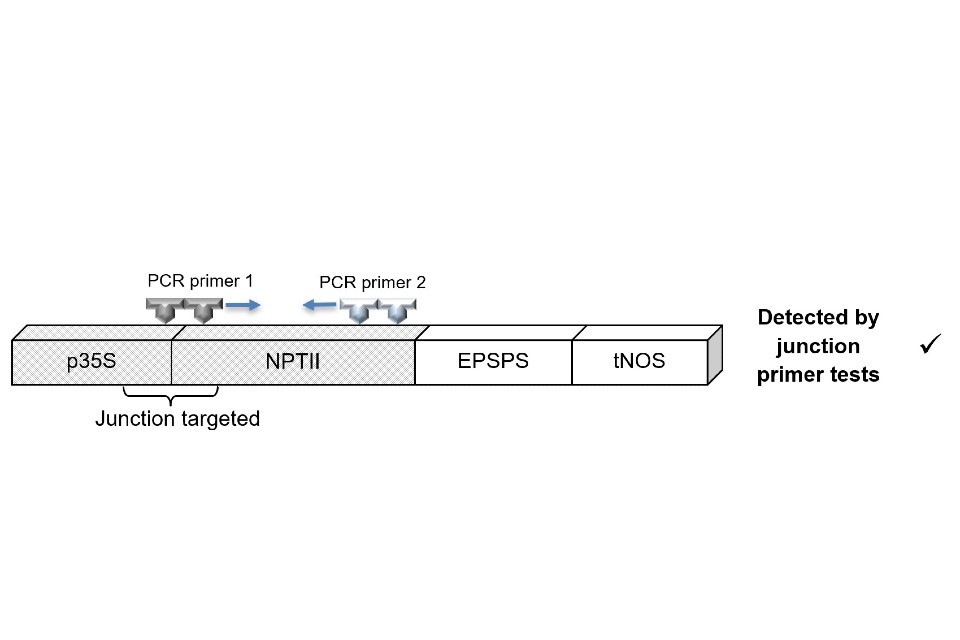
A bar with segments labelled from left to right: p35S, NPTII, EPSPS, tNOS with p35S and NPTII coloured as "Junction targeted" and outside the bar reads Detected by junction primer tests with a check mark
In figure 2 the p35S and NPTII elements are present in the construct, but not detected by the test, as they are not adjacent.
Figure 2
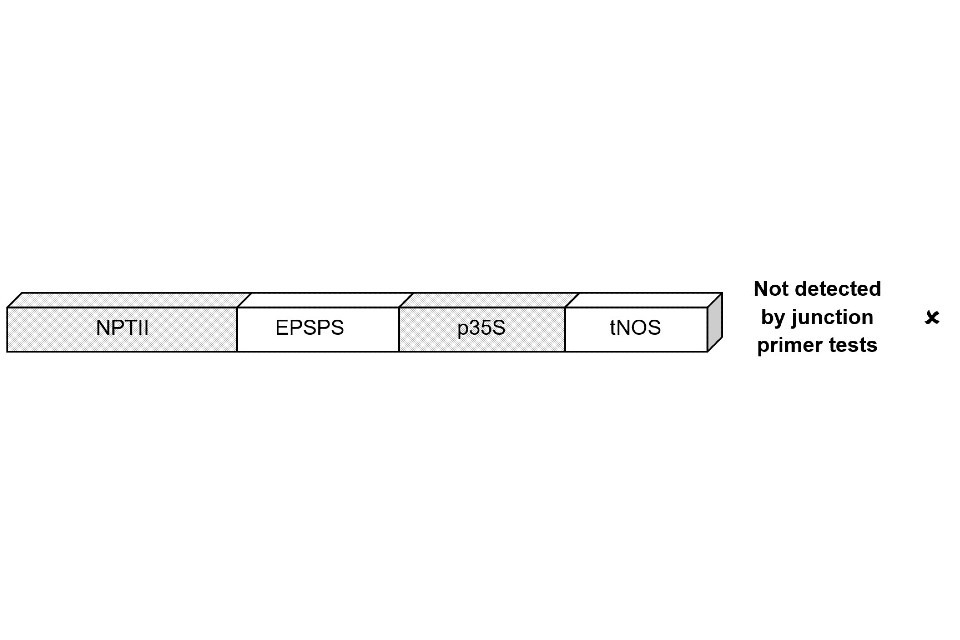
A bar with segments labelled from left to right: NPTII, EPSPS, p35S, tNOS with p35S and NPTII colour. Outside the bar reads not detected by junction primer tests with a cross mark
Despite the p35S and NPTII elements being present in both constructs, the junction-spanning test only returns a positive result for the construct in figure 1. Consequently, such junction-spanning tests could fail to detect a range of GM lines containing p35S or NPTII.
Companies that use junction-spanning PCR tests should include the names of the specific genetic elements, constructs or events that are targeted on the certificate.
Audit summary reports
After completing a seed audit, the auditor submits a detailed report to Defra showing the:
- names of varieties imported or produced by the seed company
- assurances provided by them for each seed lot or batch
See recent .
Legal definitions
Genetically modified organisms and genetic modification are defined in:
- section 106 of the Environmental Protection Act 1990
- Genetically Modified Organisms (Deliberate Release) Regulations 2002
- Genetic Technology (Precision Breeding) Act 2023
Contact
Contact the GMI:
- for information about the seed audit programme
- for timings of seed audits
- if you have concerns or information about unauthorised GMOs that you believe may have been released in England
Email: gm-inspec@apha.gov.uk
GM Inspectorate
Animal and Plant Health Agency
Sand Hutton
York
YO41 1LZ
North Yorkshire
United Kingdom
Updates to this page
-
Updated the GMI seed audit summary reports document.
-
Updated the GMI seed audit summary reports document.
-
Updated the GMI seed audit summary reports document.
-
Updated information on PCR-based tests.
-
Updated the GMI seed audit summary reports document.
-
Updated the GMI seed audit summary reports document.
-
Updated content for the 2024 audit programme covering information about the introduction of the Genetic Technology (Precision Breeding) Act.
-
Updated the GMI seed audit summary reports document.
-
Updated the GMI seed audit summary reports document.
-
Updated the GMI seed audit summary reports document.
-
Updated GMI seed audit summary reports document.
-
Updated GMI seed audit summary reports document.
-
Updated GMI seed audit summary reports document.
-
Updated GMI seed audit summary reports document.
-
Updated to the content for 2022 audit programme covering amendments to legislation and rearranged the guidance for a more logical flow of information. Separated out Figure 1 into 2 separate figures. Out of date link to questionnaires removed.
-
Updated the GMI seed audit summary report.
-
Removed guidance for seed companies document and added content to a new section - importing and producing seeds. Updated the content for 2021 audits programme.
-
Updated document on recent Genetic Modification Inspectorate seed audit summary reports.
-
Updated document: recent Genetic Modification Inspectorate seed audit summary reports.
-
Updated recent GMI seed audit summary reports document
-
Clarification that GM seed audits are carried out by, or on behalf of, the competent authorities of the UK.
-
Guidance for seed companies updated
-
GMI seed audit report updated
-
Recent GMI seed audit summary report updated
-
Recent GMI seed audit summary report updated.
-
GMI Seed Audit Summary report updated
-
Recent GMI seed audit summary report published.
-
Published summary report for 2018 audit programme
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 updated
-
Seed audit summary 2017 added
-
Updated document: guidance for seed companies
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2015/16 updated
-
Seed audit summary 2014/15 updated. Seed audit summary 2015/16 added.
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary 2014/15 updated
-
Seed audit summary table 2014/15 updated
-
Summary report for 2014/15 published
-
First published.