Treating paracetamol overdose with intravenous acetylcysteine: new guidance
New simplified guidance on treating paracetamol overdose with intravenous acetylcysteine including an updated treatment nomogram.
Article date: September 2012
Paracetamol overdose can result in liver damage which may be fatal. Intravenous acetylcysteine is the antidote to treat paracetamol overdose and is virtually 100% effective in preventing liver damage when given within 8 hours of the overdose. After this time efficacy falls substantially, affording only a very limited window of time in which to successfully prevent serious hepatotoxicity.
New simplified guidance on the treatment of acute paracetamol overdose with acetylcysteine is now in place, following an evidence-based review by the Commission on Human Medicines (CHM).
Review findings
Previously, healthcare professionals treating paracetamol overdose were advised to assess for risk factors of hepatotoxicity such as chronic alcohol consumption, co-medications and poor nutritional intake. This resulted in two lines on the treatment nomogram – 1 for patients with risk factors and 1 for those without. The CHM review found that the evidence base to support the use of risk factors was poor and inconsistent, and that many of the risk factors for hepatotoxicity were imprecise and difficult to determine with sufficient certainty in clinical practice. By removing the need to assess risk factors for hepatotoxicity, the approved indication for acetylcysteine is greatly simplified to a single line on the paracetamol overdose treatment nomogram (figure 1).
In the past there were also a substantial number of reports of administration errors with intravenous acetylcysteine, some of which had the potential to result in significant harm. A major contributory factor for these errors was the complex dosing regimen for intravenous acetylcysteine. The CHM recommended a number of measures to reduce the incidence of administration errors, most notably the introduction of weight-based dosage tables for adults and children to remove the need to calculate the dose.
The majority of common dose-related adverse reactions occur within the first hour of the initial infusion of acetylcysteine. Sufficient evidence of efficacy is available to support extending the time of the initial infusion from 15 minutes to 60 minutes in order to reduce the incidence of adverse reactions.
There are now no specific contraindications to acetylcysteine in the treatment of paracetamol overdose, including known hypersensitivity to any of the ingredients in the product. Even if a patient has a history of a previous reaction to intravenous acetylcysteine, the benefits of acetylcysteine outweigh the risks in such cases, and patients should receive treatment. Any ‘hypersensitivity-like’ reactions ascribed to acetylcysteine are likely to be anaphylactoid in nature; ie, they are not immunologically mediated and therefore may not occur on repeated exposure.
Advice for healthcare professionals:
- all patients with a timed plasma paracetamol level on or above a single treatment line joining points of 100mg/L at 4 hours and 15mg/L at 15 hours after ingestion should receive acetylcysteine (Parvolex or generics) based on a new treatment nomogram, regardless of risk factors (see figure 1 below)
- where there is doubt over the timing of paracetamol ingestion including when ingestion has occurred over a period of one hour or more – ‘staggered overdose’ – acetylcysteine should be given without delay (the nomogram should not be used).
- administer the initial dose of acetylcysteine as an infusion over 60 minutes to minimise the risk of common dose-related adverse reactions
- hypersensitivity is no longer a contraindication to treatment with acetylcysteine
- new weight-based dosing tables and a technical information leaflet (TIL) to help calculate the dose of acetylcysteine and infusions to minimise the risk of dosing errors are available to download
- a model patient discharge leaflet for patients who have taken a paracetamol overdose but who are not treated with acetylcysteine is available to download
Please report serious adverse reactions to acetylcysteine or any other medicine to the MHRA through the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
Figure 1. New treatment nomogram for paracetamol overdose
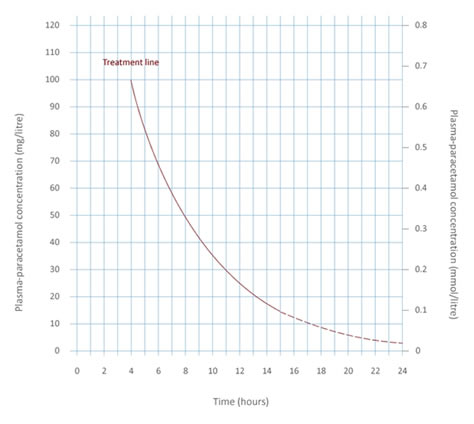
Image of new treatment nomogram for paracetamol overdose.
Further information:
MHRA webpage on new guidance for the treatment of paracetamol overdose with acetylcysteine
Letter sent to healthcare professionals in September 2012
Article citation: Drug Safety Update September 2012, vol 6, issue 2: A1