Spironolactone and renin-angiotensin system drugs in heart failure: risk of potentially fatal hyperkalaemia—February 2016 article
Monitoring of blood electrolytes is essential in patients coprescribed a potassium-sparing diuretic and an angiotensin converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) for heart failure.
Reminder for healthcare professionals:
- concomitant use of spironolactone with ACEi or ARB increases the risk of severe hyperkalaemia, particularly in patients with marked renal impairment, and should be used with caution
- use the lowest effective doses of spironolactone and ACEi or ARB if coadministration is considered essential
- regularly monitor serum potassium levels and renal function
- interrupt or discontinue treatment in the event of hyperkalaemia
- the same advice applies regarding concomitant use of eplerenone with ACEi or ARB in heart failure
- suspected adverse reactions should be reported to us on a Yellow Card
Risk of hyperkalaemia with spironolactone
Spironolactone is indicated in patients with congestive heart failure. It is a competitive aldosterone antagonist that increases sodium excretion while reducing potassium loss at the distal renal tubule. This mechanism of action means that hyperkalaemia can occur, particularly in patients with impaired renal function. Spironolactone should not be used in patients with severe renal impairment or pre-existing hyperkalaemia.
Risk of hyperkalaemia with renin-angiotensin system drugs
ACEi are mainly indicated in patients with hypertension or heart failure. ARBs are also indicated in hypertension and some are also indicated in heart failure. Recognised side effects of treatment with an ACEi or ARB include renal dysfunction and an increase in serum potassium. Risk factors for hyperkalaemia, such as renal insufficiency and diabetes mellitus, are more common in patients who require treatment with ACEi or ARB. Dehydration may also increase the risk of renal dysfunction leading to hyperkalaemia. Hyperkalaemia has been estimated to occur in between 1 in 100 and 1 in 1000 patients who take an ACEi or ARB.
Reporting of cases of hyperkalaemia
A recent coroner’s case reported to us described a case of fatal hyperkalaemia in a patient with heart failure, diabetes, and chronic renal failure who was being treated with several medicines including spironolactone. A low-dose ACEi was subsequently added for treatment of increased blood pressure. A few days later, the patient was admitted to hospital with severe hyperkalaemia and acute-on-chronic renal failure and subsequently died.
Between January 1998 and December 2015, we have received 82 UK spontaneous reports of abnormal blood potassium in patients using spironolactone as well as an ACEi (n=63) or ARB (n=25), 70 of which describe hyperkalaemia. 3 patients taking spironolactone and ACEi had a fatal outcome.
The number of cases reported for concomitant use of spironolactone and ACEi has increased from 1999, peaking 2001–05, and has started rising again in the past 2 years (see figure). During the period from 1982 (when the first report of hyperkalaemia with this combination of medicines was received) to 1998, only 7 cases of hyperkalaemia with spironolactone and an ACEi or ARB were reported.
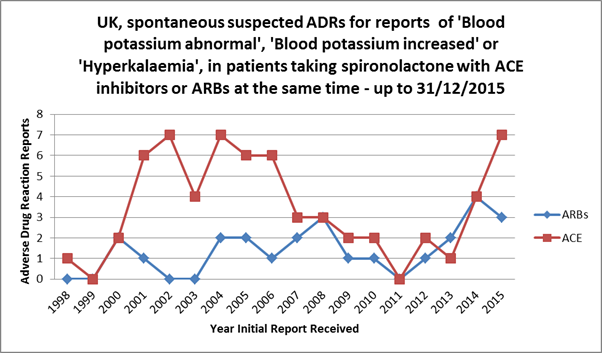
Image of graph showing Reported UK spontaneous cases of abnormal or increased blood potassium, or hyperkalaemia, in patients using spironolactone and ACEi (63) or ARB (25) at the same time for the period January 1998 to December 2015.
Figure: Reported UK spontaneous cases of abnormal or increased blood potassium, or hyperkalaemia, in patients using spironolactone and ACEi (63) or ARB (25) at the same time for the period January 1998 to December 2015. Note: some cases included both ACEi and ARB use with spironolactone.
In 1999, the Randomised Aldactone Evaluation Study (RALES)[footnote 1] reported a relative risk reduction in death for patients treated with spironolactone compared with placebo, in addition to standard therapy (including ACEi) if tolerated. The incidence of hyperkalaemia in RALES was low. In 2004, a study in the USA and Canada found an association between spironolactone use and hyperkalaemia-associated morbidity and mortality in patients treated with ACEi who had been recently admitted to hospital for heart failure.[footnote 2] This observation was thought to be due to differences between conditions and baseline patient characteristics in the RALES trial compared with those seen in routine clinical practice.[footnote 2] The peak of UK reporting after 1999 possibly reflects increased use of spironoloactone and ACEi following RALES.
The recent increase in reporting has coincided with the outcome of a European review on dual blockade therapy with ACEi and ARB. This review concluded that combination use of ACEi and ARB (which both inhibit the renin-angiotensin system) is not recommended because of an increased risk of hyperkalaemia, hypotension, and impaired renal function. The recent increase in number of UK cases reported could reflect an increase in coadministration of spironolactone and ACEi or ARB, or it could represent stimulated reporting due to increased awareness of the risks.
Footnote: article clarification, December 2016
Note that this article was clarified in December 2016. Further information can be found here.
Article citation: Drug Safety Update volume 9 issue 7 February 2016: 2.
-
Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–17. ↩
-
Juurlink D, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004; 351: 543–51. ↩ ↩2
Updates to this page
-
Republishing.
-
DrugSafetyUpdate: HIV booster and steroid coadministration: risk of systemic corticosteroid-related adverse effects…
-
First published.