Human papillomavirus immunisation programme
Second year safety review
Article date: October 2010
The human papillomavirus (HPV) immunisation programme has now completed its second successful year and is ready to enter its third. With at least 4·5 million doses of Cervarix administered up to the end of July 2010 to girls aged 12 to 18 years across the UK, the vaccine has been shown to have an excellent safety profile. Most Yellow Card reports have related either to the signs and symptoms of recognised, minor side effects listed in the product information, or to the injection process and not the vaccine itself (ie, psychogenic in nature).
Safety monitoring strategy
Our proactive and transparent approach to safety monitoring for the HPV vaccine during its first 2 years of use has enabled real-time, scientifically robust analysis of vaccine safety. This strategy involved daily analysis of all suspected adverse reactions, and weekly publication of safety reports (see www.yellowcard.gov.uk and www.mhra.gov.uk/hpvvaccine). The approach has provided reassurance on events likely to be coincidental to the vaccine, and helped to minimise unfounded concerns among parents and teenagers. As with all vaccines and medicines, the MHRA will continue to keep the safety of Cervarix vaccine under close review.
Cervarix: current safety profile
Up to the end of July 2010, we received 4703 Yellow Cards, including 10,410 adverse-reaction terms, in association with Cervarix (including reports in which the brand of HPV vaccine was not stated by the reporter). The reporting profile is very much in line with that of the first year of the programme (see Drug Safety Update October 2009), and the total number of reports received over the past 2 years of the programme is consistent with that expected.
Number of reports received per month September 2008 to July 2010:
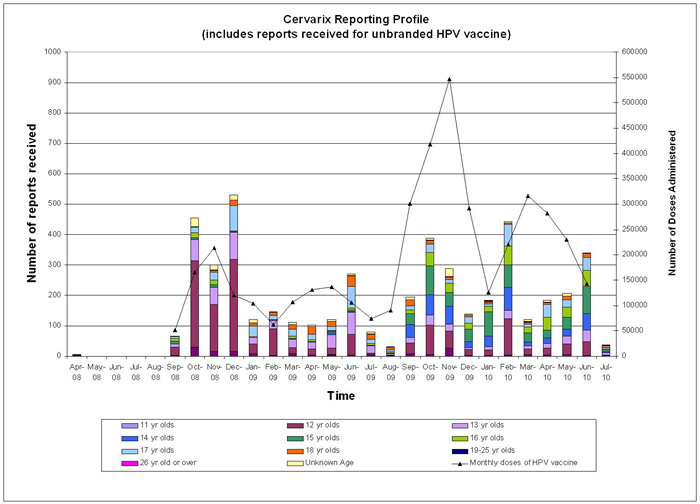
Image of graph showing number of reports received per month September 2008 to July 2010.
As expected, most reports to date have been in girls aged 12 to 13 years and have been submitted by nurses (mostly school nurses).
The type of adverse reactions reported to the Yellow Card Scheme:
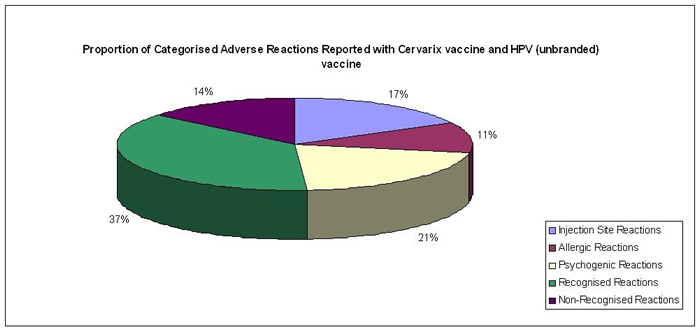
Image of pie graph showing types of adverse reactions reported.
Most reports continue to be related to minor reactions already recognised and listed in the product information, or to ‘psychogenic’ events such as faints, which are not due to the vaccine per se but to the injection process. ‘Psychogenic’ events are relatively common in adolescent immunisation programmes. More information on the proportion of categorised adverse reactions reported can be found at www.mhra.gov.uk/hpvvaccine.
Other adverse events assessed
All reported adverse events which are not currently recognised as possible side effects have been assessed. Most related either to isolated cases or small clusters of cases that are naturally occurring medical conditions among teenage girls. The available information does not indicate any consistent clinical pattern that may suggest an association with Cervarix. Given that at least 4·5 million doses have been administered to date, it is inevitable that many clinical events will occur in temporal association with vaccination, regardless of a causal association. Such events may be due to underlying/undiagnosed concurrent illness unrelated to vaccination.
Chronic fatigue syndrome (CFS) and associated conditions
CFS is a condition that occurs naturally among the population of girls vaccinated. Among the number of girls immunised so far in the UK, at least 100 cases of CFS would have been expected to have occurred by chance alone. Accounting for various levels of possible under-reporting to the Yellow Card Scheme, the reports so far in the UK do not indicate that Cervarix is a cause of CFS.
Guillain-Barré syndrome
Five cases of Guillain-Barré syndrome (GBS) have been reported via the Yellow Card Scheme so far, with no consistent pattern of clinical presentation. Given the expected background incidence of GBS in the vaccinated population, our analyses have suggested that the reported cases are consistent with these being coincidental with vaccination.
Encephalitis
The five cases of encephalitis reported so far among the number of girls immunised, does not exceed the overall number of cases we would normally expect in the absence of vaccination. As with CFS and GBS, encephalitis naturally occurs in the population and is most often caused by a preceding viral infection. There is no suggestion at present that the vaccine can cause encephalitis. As with other reported events, this will remain under review.
Summary
The government’s independent expert advisory Commission on Human Medicines (CHM) has scrutinised our 2-year safety analysis and agreed that the number and nature of suspected adverse reactions received since the vaccination programme started is fully in line with what we expected to receive at this time. CHM advised that no new or serious risks have been identified during use of Cervarix in the UK, and that the balance of benefits and risks remains positive. We will continue to monitor closely the safety of Cervarix during routine use in the UK.
Have you reported a Yellow Card for the HPV vaccine?
Thank you once again to those who have contributed to the success of this monitoring by sending us Yellow Cards for Cervarix. We encourage healthcare professionals involved in immunisation to please continue to help us monitor the safety of Cervarix by reporting any suspected adverse reactions associated with the vaccine via the Yellow Card Scheme. You can report online at www.yellowcard.gov.uk or by post (paper Yellow Cards are available at the back of the British National Formulary or call us on 0800 731 6789). Remember that parents and vaccinees can also report suspected side effects on a Yellow Card.
Further information is available in a safety report available online at www.mhra.gov.uk/hpvvaccine
Article citation: Drug Safety Update Oct 2010, vol 4 issue 3: H2.