DNA contamination controls: laboratory (accessible)
Published 26 October 2023
Issue 1
Publication date October 2023
This document is issued by the Forensic Science Regulator in line with Section 9(1) of the Forensic Science Regulator Act 2021.
© Crown Copyright 2023
The text in this document (excluding the Forensic Science Regulator’s logo, any other logo, and material quoted from other sources) may be reproduced free of charge in any format or medium providing it is reproduced accurately and not used in a misleading context. The material must be acknowledged as Crown Copyright and its title specified.
This document is not subject to the Open Government Licence.
1. Introduction
1.1.1 For the purposes of this guidance, contamination is defined as the undesirable introduction of DNA, or biological material containing DNA, to an item/exhibit or sample which is to be examined/analysed. DNA contamination in laboratory activities is distinct from the adventitious transfer of biological material to an exhibit, often referred to as background DNA, that can occur, usually prior to the exhibit or sample being recovered and before a controlled forensic process is started.
1.1.2 The principal sources of DNA contamination in the laboratory are:
a. personnel to the exhibit/DNA sample;
b. contaminated equipment or consumables to the exhibit/DNA sample; and
c. exhibit to exhibit or DNA sample to DNA sample.
1.1.3 Contamination may occur as follows:
a. directly, also described as primary transfer, for example, saliva or dandruff from an examiner falling on to an exhibit; or
b. indirectly, also described as secondary or tertiary transfer, for example, biological material present on the outside of exhibit packaging being transferred on to the gloves of an examiner who opens the package and fails to change their gloves before handling the contents, resulting in the indirect transfer of contamination to the exhibit.
1.1.4 It is recognised that DNA contamination incidents cannot be eliminated completely, given the prevalence of human DNA within the living and working environment, and the issue is exacerbated by the increasing sensitivity of DNA analytical techniques. Therefore, an effective DNA contamination control process requires a combination of approaches both to minimise the risk of occurrence and to maximise the ability to detect contamination when it does occur.
1.1.5 Laboratory contamination control measures fall into two core areas of activity.
a. Preventative measures including:
i. minimising the chance of contamination occurring by, for example, staff using barrier clothing/PPE;
ii. ensuring effective separation of exhibits from different scenes and individuals;
iii. restricting access to areas containing exhibits and consumables;
iv. cleaning laboratory surfaces;
v. using consumables which are free from detectable human DNA (for example consumable compliance with PAS 377:2023 [1] or ISO 18385 [2]; and
vi. ensuring that practitioners are aware of contamination risks and trained in the use of contamination control measures.
b. Detection of contamination primarily entailing:
i. Use of negative control samples at appropriate stages of the DNA analysis process, such as DNA extraction and amplification.
ii. comparing DNA profiles generated from items against elimination databases containing DNA profiles from personnel from whom there is a significant risk of contamination;
iii. cross-checking profiles within the same batch of samples and from different batches of samples processed within the same laboratory; and
iv. investigating unexpected results, including regular review of load failure reports.
2. Scope
2.1.1 This document provides guidance and recommendations on anti-contamination measures for the analytical phase of investigations, namely the control and avoidance of contamination in laboratory activities involving DNA evidence recovery and analysis.
2.1.2 The requirements set out in section 103 of the Code should be referred to when considering contamination controls for DNA analysis.
3. Terms and definitions
3.1.1 The terms and definitions set out in the statutory Code also apply to this guidance.
3.1.2 The interaction of the Forensic Science Regulator’s guidance together with the DNA consumable standards PAS 377:2023 [1] and BS ISO 18385:2016 [2] is shown in Figure 1.
Figure 1: Interaction of prevention and detection principles across the DNA workflow from recovery of material to analysis
| Recovery – e.g. Incident scene examination | Forensic Grade Consumables | Laboratory Analysis | ||
|---|---|---|---|---|
| Prevention | Contamination Controls FSR-GUI-0016 |
PAS 377:2023 ISO 18385:2016 |
Contamination Controls FSR-GUI-0018 |
|
| Detection | Elimination Databases | Elimination Databases | Elimination Databases |
3.1.3 A glossary of terms and abbreviations used in this guidance can be found at the end of this document.
4. The word ‘shall’ has been used in this document where there is a corresponding requirement in the Code, the word ‘should’ has been used to indicate generally accepted practice, and the word ‘may’ has been used for recommendations. Recommendations have been used to indicate what ideal practice is when it is practicable.
5. Methods and procedures
(ISO/IEC 17025:2017 sec. 7.2)
5.1 Overview
5.1.1 The Code (section 30.6.1) requires that once a method has been designed or determined, there shall be an assessment to identify any risks or potential risks to the criminal justice system (CJS) related to its use.
5.1.2 This assessment should include identification of aspects of the process that represent a significant risk in terms of contamination. If necessary, amendments to the method should be made to reduce both the opportunities and mechanisms by which contamination could occur.
5.2 Reducing risk by design
Generic
5.2.1 For DNA processes segregation of activities can significantly reduce the risk of contamination, this includes the following.
a. Segregation of the examination, DNA recovery, and extraction of casework from reference samples (i.e. pre-PCR processes). This could be through the use of separate laboratories and dedicated equipment for processing casework and reference samples. Practitioners should not enter a dedicated casework pre-PCR processing area after working in a reference sample pre-PCR processing area(s) on the same day unless they remove PPE dedicated to the reference processing area upon exiting, and apply new PPE dedicated to the casework processing area upon entering.
b. Separation of examination of high DNA yield items (for example, heavily bloodstained or semen stained items) and low DNA yield items (for example, those for trace DNA) where possible.
c. Automate manual processes through robotic handling devices to minimise contamination risks through practitioner handling.
d. Minimise contamination by polymerase chain reaction (PCR) product of upstream processes by:
i. segregation of pre-PCR (DNA amplification stage) and post-PCR processing. Practitioners should not move between pre- and post-PCR laboratories/activities without changing PPE/barrier clothing.
ii. Use of a validated, fully automated, enclosed DNA analysis system in which samples, once loaded, are automatically extracted and amplified without human intervention. Validation of such systems should include assessment of contamination vulnerability in order to inform batch size, spatial arrangements and batch monitoring requirements, for example.
Batch processing (DNA sample to DNA sample contamination)
5.2.2 To ensure efficient operation, DNA samples are routinely processed in batches. Reducing the risk of sample-to-sample contamination is primarily achieved by minimising the opportunities for occurrence through process design, including the following:
a. Minimising the time that samples are held in open receptacles such as unsealed microtiter plates and uncapped microfuge tubes.
b. Minimising the opportunity for sample to sample transfer by keeping batch sizes manageable and processing casework samples and reference samples on separate microtiter plates.
c. Designing robotic handling so that samples are processed in a sequential fashion, ensuring that no sample is moved above another unprotected sample for example, whilst being transferred in a pipette tip.
d. Paying careful attention to the detailed programming of sample manipulation steps in automated systems such as pipetting and centrifugation, both in terms of transferring samples and in mixing, to avoid splashing, dripping or the creation of aerosols.
e. Storing samples in microtiter plates, with a watertight seal and including a spin stage to remove any liquid from the internal surface of the seal prior to re-sampling.
f. Using effective, validated cleaning procedures [2] to minimise the risk of a build-up of background levels of DNA over time.
g. Having a system in place for labelling and disposal of used plates and tubes to prevent accidental re-use.
Process flow
5.2.3 Access to DNA clean areas including entrance lobbies should be controlled and only permitted for individuals who have submitted a DNA sample for inclusion on the forensic unit’s elimination database.
5.2.4 To reduce the potential for introduction of contaminants, DNA clean areas should not be used as a through corridor and the number of people accessing the area should be kept to a minimum.
5.2.5 Samples and associated materials such as tube racks should ideally be moved from pre-PCR areas to post-PCR areas by means of a service hatch, with separate in and out doors, as a means of preventing contamination of pre-PCR areas with PCR product. There should be minimal movement of items in the reverse direction and only after thorough cleaning.
5.3 Evaluating new processes for contamination control measure effectiveness
5.3.1 Once a new or modified process intended for introduction into casework has been developed, it shall be validated prior to its implementation in accordance with the Code (section 30). This includes an assessment to identify any risks or potential risks to the CJS related to its use that it has not been possible to eliminate by process engineering as detailed above. For any processes that are linked to DNA analysis, this risk assessment should include the following.
a. Consideration of contamination risks, including defining the acceptable levels in:
i. the end user requirements; and
ii. the acceptance criteria.
b. Evaluation of the impact of introducing technology that increases the sensitivity of the DNA analysis process compared with existing processes, as this also inevitably increases the chance of detecting contamination.
5.3.2 The following examples of elements of validation exercises are useful for evaluating contamination risk:
a. testing automated pipetting systems by processing ‘chequer-boards’ – microtiter plates (or equivalent plate or tube layout used by the system) where negative controls and positive samples are arranged alternately across the plate – the positive samples here should represent the ‘worst case scenario’, that is, the highest sample concentration expected to be encountered using the system;
b. evaluations of all negative controls on each batch of samples run throughout the validation exercise, and comparison with existing systems;
c. evaluation of all positive samples on each batch of samples run throughout the validation exercise to assess whether minor components could be from contamination, and comparison with existing systems;
d. checking for operator contamination throughout the validation exercise;
e. checking for contamination from consumables throughout the validation exercise – evaluating all new consumables used in the process and designing suitable quality assurance/control (QA/QC) procedures;
f. investigating the root cause where contamination is identified in the validation work.
5.3.3 Where contamination is identified in the validation exercise modifications should be made to the process to minimise the risk of recurrence and the relevant aspects of the validation study repeated.
5.3.4 Once a new system or method has been implemented, a post-implementation review should be carried out after an appropriate interval, including detailed evaluation of any contamination observed and any resulting requirements for modifications to procedures and revalidation (Code section 30.3.11).
6. Laboratory design and layout
(ISO/IEC 17025:2017 sec. 6.3)
6.1 Structure
6.1.1 In essence a DNA clean area is a room or specified enclosed area or cabinet that can be easily cleaned, and that is kept clean using validated cleaning procedures. This is facilitated by, for example, avoiding dust-traps. Ideally this should be compliant with BS EN ISO 14644:2015 [3].
6.1.2 The ideal set up for a DNA clean area should include:
a) A designated area for gowning up, ideally a separate lobby area.
b) Controlled access (see 4.2.3) to the DNA clean area through the lobby (designated gowning) area.
c) Walls of smooth finish, sealed and resistant to degradation from frequent cleaning. The active agent, corrosive nature and downstream effects from the cleaning materials used need to be understood; surfaces need to be resistant to degradation as a result of frequent contact with the cleaning reagents.
d) Readily cleanable, laboratory standard flooring material, for example fully sealed vinyl, and it is recommended that it continues part way up adjoining walls to facilitate cleaning.
e) Use of curved coving at the junctions between the floors, walls and ceilings to avoid crevices that are difficult to clean.
f) Sealed window glazing to prevent draughts and ideally the sills should be sloped with an easily cleanable surface. Where blinds are required, ideally these should not be on the inside of the window .
g) Ceilings made of a material resistant to degradation from frequent cleaning, for example, laminated tiles of smooth finish.
6.2 Furniture
6.2.1 Bench surfaces should be sealed and of laboratory grade, resistant to chemicals such as strong acids, alkalis and solvents, and withstand frequent cleaning without staining or deteriorating. White bench surfaces are recommended.
6.2.2 Laboratory chairs should be height adjustable and covered in a non-porous material such as vinyl, which can withstand frequent cleaning without staining or deteriorating.
6.2.3 Bench workstation drawer units should provide sufficient storage capacity to enable bench surfaces to be kept clear, other than large or frequently used equipment.
6.3 Lighting
6.3.1 Lighting meeting the ISO 8995-1:2002 standard [2] is recommended, ideally in recessed units finished flush with the ceiling (ingress protection rated IP65) in order to provide a smooth waterproof surface for cleaning purposes.
6.3.2 The optimum lighting level should be a minimum of 1,000 lux at bench level, supplemented by variable task lighting at the point of examination, whilst accounting for Health and Safety Executive (HSE) requirements for lighting at work [3].
6.4 Air quality and air flow
6.4.1 Air movement within and between rooms should be managed, with measures taken to minimise the risk of contamination from background DNA.
6.4.2 Airborne particulate cleanliness equivalent to ISO 14644-1:2015 [3] Class 7 is recommended.
6.4.3 A minimum airflow of 20 times whole room replacement per hour is recommended. Higher replacement rates may be required to maintain ambient temperature in laboratories utilising equipment such as sequencers with high heat outputs.
6.4.4 Filtration of input air to all DNA clean areas by suitable high efficiency particulate air (HEPA) filtration units is recommended. This can be achieved, for example, by utilising a HEPA-filtered clean air cabinet as a DNA clean area.
6.4.5 Management of air flow systems is essential to prevent output of unfiltered air from post-PCR areas from re-entering pre-PCR areas including search areas.
6.4.6 Air movement should be controlled to minimise adverse draughts or uncontrolled turbulence.
6.4.7 Samples likely to generate airborne material that may be a source of DNA (e.g. blood flakes) during their examination should be examined in a clean air cabinet, unless the bulkiness of the item renders this impractical. Under these circumstances the item should still be examined in an area where that reduces the risk of contaminating other items.
6.5 Air Pressure Regime
6.5.1 An ideal air pressure regime is outlined in figure 2, where ‘0’ denotes ambient pressure outside the building, ‘+’ denotes positive air pressure, and ‘++’ is positive air pressure higher than +. This prevents accidental ingress of contaminating material into a pre-PCR DNA clean area and egress of PCR product from a post-PCR area.
Figure 2: Schematic of relative air pressures for laboratory housing DNA clean rooms/areas
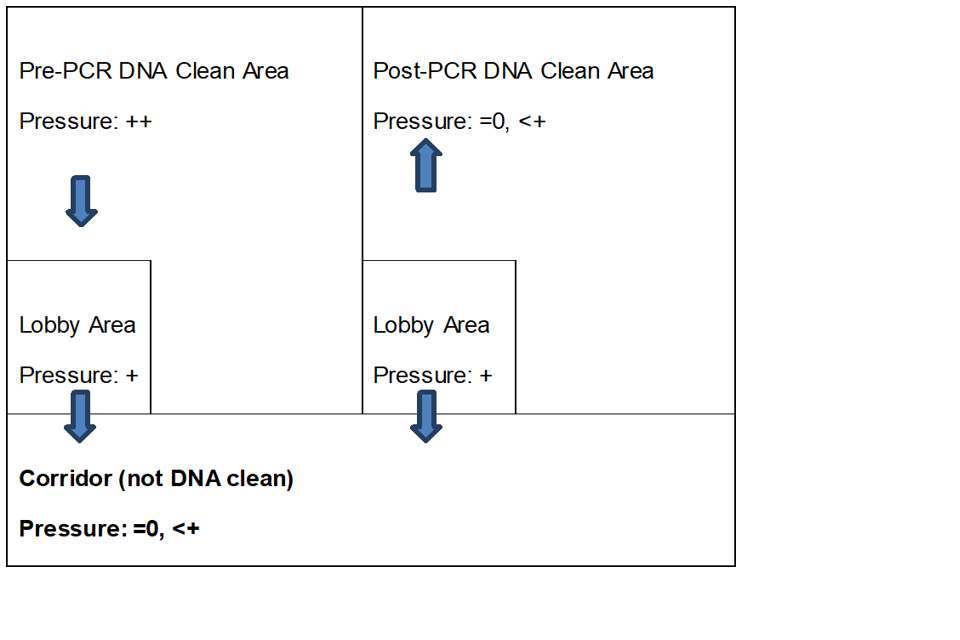
The schematic shows that the pressure in the lobby area should be higher than in the not DNA clean corridor, the pressure in the pre-PCR DNA clean area should be higher than in the lobby area, and the pressure in the post-PCR DNA clean area should be 0, less than in the lobby area.
6.6 Lobby area
6.6.1 The lobby should ideally have interlocking doors to minimise ingress of contaminants, and the door from the outside corridor should open inwards into the lobby. If a designated gowning area is used in place of a separate lobby then the risk of transfer of DNA from the gowning area to the DNA clean area should be taken into account the forensic unit’s cleaning and environmental monitoring regimes.
6.6.2 Lobby areas for DNA clean laboratories should contain a hand-wash sink with hot and cold water, dispensers for soap and disposable towels.
6.6.3 Lobby areas for containment laboratories may use sanitisation gel in preference to hand-wash sinks.
6.6.4 Lobby areas should have coat pegs for hanging up laboratory coats and storage shelves/cupboards for consumables, equipment such as pens, and PPE.
6.7 Other
6.7.1 Laboratory equipment and stationery should be dedicated to each particular DNA clean area, and to each individual workstation within a given area.
7. Contamination prevention
7.1 Receiving items/exhibits and initial examination – DNA clean areas
7.1.1 As required in section 35.2.1 of the Code, there shall be a documented policy for receipt of items/exhibits, including the actions to take should there be any issues with the integrity of the packaging or package seals. Where there is doubt about the suitability of the item/exhibit for DNA analysis as a result of compromised packaging the commissioning party shall be informed (see ISO/IEC 17025, section 7.4.3).
7.1.2 Where a decision is made to proceed with examination of an item/exhibit with compromised packaging all relevant parties shall be informed, this includes practitioners working on the case within the forensic unit, external forensic units commissioned to carry out work on the item/exhibit of sub-exhibits, and commissioning parties (see ISO/IEC 17025:2017, section 7.4.3).
7.1.3 Consideration should be given to wiping down the outside of plastic exhibit packaging with an appropriate cleaning solution.
7.1.4 Items/exhibits that may require DNA analysis shall be examined in suitable conditions that do not affect the validity of results (ISO/IEC 17025:2017, section 6.3.1) which should be taken to mean in a DNA clean area.
7.1.5 Gloves should be changed or cleaned using a validated method after opening or handling the packaging in order to reduce the risk of secondary transfer. It has been shown that DNA can be transferred to the outside of evidence bags from the environment [5], therefore contact between the outside of packaging and the examination surface or the items/exhibits themselves should be avoided.
7.2 Examination outside of a DNA clean environment
7.2.1 Where it is not possible to examine items in a dedicated DNA clean area, for example, for health and safety reasons, large items or where other examinations need to be carried out before DNA samples can be taken the following activities should be undertaken:
a. the examination area should be decontaminated as completely as possible;
b. background swabs should be taken from relevant examination areas and processed as required to demonstrate the integrity of the DNA results obtained;
c. normal laboratory processes followed as far as possible, including wearing PPE/barrier clothing;
d. Inform all relevant parties, this includes practitioners working on the case within the forensic unit, external forensic units commissioned to carry out work on the item/exhibit or sub-exhibits, and commissioning parties (see ISO/IEC 17025:2017, section 7.4.3).
7.2.2 Where an exhibit has been handled in an area that is not a dedicated DNA clean area and is subsequently determined to require DNA analysis, this should be reported to all relevant parties, this includes practitioners working on the case within the forensic unit, external forensic units commissioned to carry out work on the item/exhibit or sub-exhibits, and commissioning parties.
7.2.3 Where possible, profiles generated from items/exhibit examined outside of a DNA-clean area should be checked against all practitioners who handled the unpackaged exhibit and, if necessary, against previous casework undertaken in the area.
7.2.4 Any resulting report and/or statement shall explicitly refer to the contamination risk and any deviation from normal process (see ISO/IEC 17025:2017, section 7.4.3), for example, where items/exhibits have been handled outside of a DNA clean environment or where it has not been possible to carry out relevant profile elimination checks and the significance of this in the context of the case. This is a particular issue when items from historic cases are re-sampled.
7.3 Personnel
(ISO/IEC 17025:2017 ref. 6.2)
Training
7.3.1 This guidance should be introduced to all relevant practitioners and should form part of their induction training.
7.3.2 Issues relating to contamination risks and their avoidance in specific processes and methods should be an integral part of practitioner training.
7.3.3 When competencies are being assessed, assessors need to ensure that the contamination risks of any process and the means of avoidance are fully understood.
7.4 Protective clothing
7.4.1 The following is a list of recommended protective clothing for anyone entering a DNA clean area.
Laboratory coats
7.4.2 Dedicated disposable/non-disposable laboratory coats covering the full upper body from the neck downwards, with full length sleeves worn and properly fastened.
7.4.3 If using reusable laboratory coats the laundering process needs to ensure that coats from pre-PCR areas are handled entirely separately from coats from post- PCR areas.
7.4.4 To avoid cross-contamination, labcoats should be changed before searching items from a different case, individual or location, and where other circumstances dictate, for example, after searching a heavily stained exhibit.
a. It is acceptable not to change laboratory coats when examining different items of clothes that have been worn at the same time by the same individual.
b. For examination and processing of volume crime samples, it is acceptable to use a lower cost alternative of wearing disposable paper aprons and sleeve covers over the laboratory coat and changing the apron and sleeve covers between cases, rather than the laboratory coat. Alternatively, the laboratory can determine the frequency of changing laboratory coats/aprons based on documented practices. For example, if the item/sample comes into contact with apron/laboratory coat/sleeves or a frequency determined and based on evidence of background DNA sampling (similar to substrate/ blanks) of sleeves/coats for specified time periods through the environmental monitoring of the regime. The changing of sleeves may be more frequent than laboratory coats and aprons as contamination is more likely to transfer via sleeves than the front of a laboratory coat/apron.
7.4.5 Labcoats should be changed or disposed of before leaving the DNA clean area.
Gloves
7.4.6 Two pairs of disposable gloves should be worn at all times in a DNA clean area, and removed when leaving the area. Disposable gloves should be powder-free nitrile gloves or another suitable alternative.
7.4.7 The wrist of the glove should cover the wrist of the laboratory coat. Where this is not possible, disposable cuffs or tape can be used to cover the gap.
7.4.8 To reduce the risk of gloves becoming contaminated they should be cleaned (using a method validated as effective at removing DNA) or the outer pair changed after:
a) Handling exhibit packaging;
b) touching the face;
c) touching door handles or other frequently touched surfaces;
d) touching irregularly cleaned surfaces, such as the floor;
e) touching PPE (e.g. putting on a labcoat that has previously been used)
7.4.9 Gloves (outer) should be changed between the examination of different items or between batches of DNA samples.
7.4.10 Gloves do not need to be changed between exhibits whilst these are still in their packaging, for example, when transporting intact exhibit bags to new locations.
7.4.11 Practitioners may also change or wipe gloves during item examination or when processing a batch of DNA profiling samples, for example when moving from examining the outside of a jacket to examining the inside.
Face masks
7.4.12 When examining exhibits and handling samples, pinch nose face masks should be worn that are properly fastened and adjusted to cover the nose and mouth. Face masks used should be comfortable and not require frequent re-adjustment to avoid contaminating gloves by touching the face or mask.
Mob cap
7.4.13 A disposable mob cap or similar hair cover large enough to securely cover the head hair.
7.4.14 Where necessary, for example, with bearded individuals, additional hair cover (snoods) should be used to ensure that all facial hair is covered when used in conjunction with the face mask.
Gowning procedure
7.4.15 The gowning up procedure should be undertaken in a lobby area or in a designated area close to the entrance/exit of the DNA clean area. The sequence for gowning up should be documented and the following order is suggested:
a. mob cap, ensuring all hair is secure within the cap;
b. face mask, avoid talking until the mask is securely fitted;
c. clogs or overshoes if necessary;
d. goggles or other eye protection where necessary;
e. two pairs of gloves;
f. disposable labcoat
7.4.16 When leaving the DNA clean area the gowning up procedure should be reversed. If taking samples/tubes out of the laboratory, clean gloves should be worn to transport the samples.
7.5 Controlling bench environment
Cleaning
7.5.1 In addition to a daily cleaning regime (section 7.7.3) the search bench should be decontaminated between items, cleaning the top and sides of the bench using a detergent/cleaning solution validated as being effective at removing DNA. There may be instances, for example, where clothes are submitted from the same individual that were worn at the same time, where in the professional judgement of the practitioner not cleaning between items would not affect the interpretation of any evidence. Any variation from cleaning between items and the justification should be recorded in the case notes.
7.5.2 Each item/exhibit should be examined on a suitable disposable surface, used as an additional barrier or to collect debris, such as; a sheet of Kraft paper, plastic sheeting, or Benchkote, and should be changed between items. If the protective surface is not changed between items/exhibits this should be recorded in the relevant notes.
7.5.3 As far as possible all bench surfaces used for searching examination and processing of items/exhibits shouldbe kept clear. This makes it much easier to decontaminate the surface.
Items/exhibits left on benches
7.5.4 Items/exhibits, including those that need to dry prior to re-packaging, should not be left uncovered on the bench when not being examined. The exhibits should be covered with disposable paper sheeting.
Bench log
7.5.5 A record of the date, time and practitioner for all items/exhibits/batches examined/processed at every workstation or defined area of bench shall be kept (ISO/IEC 17025:2017, section 7.3.3). This may be required following a contamination incident, if it is thought necessary to check other items/exhibits/batches processed at the same bench or workstation.
7.5.6 As required by the Code (section 25.2.1) records of the movement and handling of items/exhibits shall be kept. These records can be electronic, for example, by means of a laboratory information management system (LIMS)/electronic management system (EMS) tracking of samples.
Paperwork
7.5.7 If paper case files are utilised then only relevant pages such as submission forms and examination instructions should be taken into a DNA clean area, preferably within a cleaned plastic wallet and wherever possible kept to one side of the work area/station.
7.5.8 Other items required for note taking (paper, pens, pencils, other stationery) should be provided within the DNA clean area and not removed.
7.6 Using DNA laboratories for activities other than casework
7.6.1 At times it may be necessary to use laboratory space for non-casework activities including for training purposes, preparation of positive body fluid controls for presumptive test reagents, and seeding of items for trials and audits.
7.6.2 A risk assessment should be undertaken to determine the risk of contamination from use of the laboratory space for the preparation of positive body fluid controls or the preparation and examination of training items. If necessary, deep cleaning and environmental monitoring should be undertaken before examining any casework items in the laboratory (see section 6.7).
7.6.3 Donors of human body fluids used for positive controls or seeding training items shall have their profiles held on the staff elimination database (SED) as there is a risk of transferring their DNA to items (Code section 103.12.1b);
Preparation and examination of items stained with body fluid for training or testing and preparation of reference controls
7.6.4 When clothing, swabs and other items are seeded with significant quantities of liquid body fluids for positive controls, training, setting up proficiency and inter- laboratory tests or for demonstration purposes, the following apply:
a. Use of an area that is not in use for DNA casework at the same time. When a number of practitioners are receiving training with seeded items, ideally a complete laboratory should be designated for the duration of the training;
b. Use of disposable pipettes and containers, or dedicated and labelled re- usable pipettes that are not used for any other purpose;
c. Keeping a record of the samples used for seeding;
d. Keeping a record on bench logs of the use of the laboratory for preparation/examination of training items or preparation of positive controls;
e. Cleaning of the benches on completion of training or preparation in line with standard processes.
Preparation of positive body fluid controls for check of presumptive test reagents
7.6.5 Where possible, semen used for presumptive test positive controls should be prepared from samples provided by a vasectomised donor, to reduce the amount of DNA present. If this is not available, the supernatant from a spermic
sample can be used. The test papers should be prepared in a non-DNA casework area.
7.6.6 Supernatants of saliva can be used as positive controls to reduce the risk of DNA contamination.
7.6.7 Blood used for positive controls should be animal blood, such as horse, rather than human.
7.7 Cleaning process
7.7.1 Each DNA clean area should have a cleaning schedule, with the frequency of cleaning dependent on the extent of use of the area and the equipment within it. As required by the Code (section 103.5.10b) cleaning log shall be maintained, this should show the daily, weekly or monthly activities undertaken as per the schedule.
7.7.2 Cleaning should follow a regime validated to provide effective DNA decontamination [2]. As disinfectants can be potent inhibitors of PCR, adversely affecting DNA results [6], the cleaning regime should also not introduce inhibition problems.
Minimum cleaning requirements
7.7.3 The following is recommended as the minimum cleaning requirements and should be undertaken using cleaning equipment dedicated solely for use in each DNA clean area.
7.7.4 Daily or after each use, clean:
a. bench work surfaces – all surfaces that may either directly or indirectly come into contact with items/exhibits (these surfaces should also be cleaned before use);
b. re-useable items such as reagent troughs, tube racks and capillary arrays; and
c. centrifuges – inside and out.
7.7.5 Before and after use, and in between the examination of different items, clean:
a. individual pieces of equipment including fibre rollers, pens, rulers, barcode scanners, low power microscopes; and
b. IT equipment (graphic pads and pens, and keyboards, etc).
c. Chairs, including height adjustment controls.
7.7.6 Weekly clean:
a. floors;
b. equipment such as microscopes, computers and all exposed cables;
c. all contact surfaces such as cupboards, door handles and fridges.
7.7.7 Routine or regularly scheduled whole area deep clean to include the areas listed above and areas not covered by other cleaning routines:
a. lights and vents;
b. walls and ceiling; and
c. insides of drawers.
7.7.8 Cleaning or replacement of air filters should be undertaken at a frequency recommended by the manufacturers.
7.7.9 For DNA clean rooms that are used on an infrequent basis, i.e. less than once a month, a deep clean should be undertaken prior to re-commencing use.
7.7.10 Where a spill or leak of biological material occurs, it should be removed using a cleaning regime validated to provide effective DNA decontamination. Depending on the circumstances and extent of the spillage it may be appropriate to undertake environmental monitoring of the affected area to provide assurance that all contamination has been removed.
7.8 Environmental monitoring
Principle
7.8.1 The principle of environmental monitoring is to undertake a programme of testing on a periodic basis to check that particular rooms or areas are DNA clean and to assess whether the decontamination policy for the area in question is both effective and has been carried out properly.
7.8.2 Results from such monitoring should be carefully assessed given that, unlike other classes of testing, DNA analysis does not typically include the additional safeguard of processing negative controls within batches.
7.8.3 Samples should be taken by swabbing selected areas and equipment that are in contact with practitioners and/or items/exhibits at all stages of DNA recovery and analysis.
7.8.4 The sampling regime should reflect the risk profile of the activities being carried out and be proportionate to the risk. For example, drying rooms in which large amounts of biological material are inevitably present should be sampled most frequently. The areas that should be sampled vary according to the function of the area and examples are given at 7.8.16.
Sampling schedule
7.8.5 The forensic unit shall have policies and procedures to monitor the ongoing effectiveness of cleaning through environmental monitoring (the Code, section 103.5.9). Routine assessment of all DNA clean work areas is required.
7.8.6 The required frequency of sampling should be evidence-based, specified in the sampling plan and reviewed through use of trend analysis. Thus an area that has basic air flow management and where many people regularly work will require more frequent cleaning and monitoring than a low throughput facility with state of the art air flow management.
7.8.7 Depending on circumstances, additional non-routine testing may be required, for example:
a. after a contamination incident has occurred;
b. after a laboratory work area has changed function; or
c. after maintenance has been completed.
7.8.8 In addition to DNA clean areas, environmental monitoring of specified areas should be undertaken where DNA contamination poses less of a risk, for example, areas where exhibits are regularly handled but remain sealed within packaging.
7.8.9 Results from monitoring these areas should be treated as an indication of background levels of DNA and used to inform the effectiveness of cleaning regimes within these areas.
Sampling and analysis
7.8.10 Swabs should be taken from an appropriate number of areas as determined in the sampling plan. Depending on the size and scope of the laboratory all relevant areas should be covered within the matrix of the sampling schedule based on risk assessment and trend analysis from monitoring results.
7.8.11 Sampling should be undertaken to optimise the recovery of DNA utilising forensic DNA grade consumables (for example, swabs moistened and/or dry as appropriate).
7.8.12 The swabs should be extracted, amplified and analysed in accordance with the commonly used method employed on samples that are processed through the particular laboratory/facility under test, including the use of positive and negative controls.
7.8.13 For each monitored area the following should be recorded:
a. the date that sampling was undertaken;
b. who undertook the sampling;
c. the number and location of areas sampled;
d. the number of failures and items/areas affected;
e. comments on allelic peaks observed (the number and peak height, whether the profiles match against the staff elimination database); and
f. corrective actions taken as detailed in section 9.5.
7.8.14 Areas to swab should be assigned different priorities, based on general experience of potential contamination hot-spots, plus specific past experience of environmental results for a specific laboratory/room.
a. Priority 1: These require swabbing in every environmental monitoring exercise, for example, workbenches, fridge freezers, keyboards, surfaces in robotic workstations, drying cabinets.
b. Priority 2: A selection of these should be included in each exercise, for example, chair seats, drawer handles.
c. Priority 3: These should be included occasionally, for example, reagent bottles.
7.8.15 Areas that are not swabbed are chemical waste bins and sharps bins.
Areas to be sampled
7.8.16 Listed below are areas that may be vectors for contamination and should be sampled. This is not necessarily an exhaustive list.
a. Automated equipment
b. Barcode scanners
c. Benches, including canopies and screens
d. Centrifuges
e. Chairs
f. Cross-linkers
g. Cupboard, drawer, door and fridge/freezer handles
h. Digital cameras
i. Drying cabinets
j. Fibre optics
k. Forceps
l. Handles of boxes of consumables
m. Heat sealers
n. Incubators
o. Instrument controls
p. Lids, tops of containers
q. PCs, keyboards, etc.
r. Pipettes
s. Racking in stores
t. Reagent bottles, including water sprayers
u. Robotic workstation surfaces
v. Safety cabinets/hoods
w. Shakers/vortex mixers
x. Stationery
y. Telephones
z. Ultraviolet lamps
7.8.17 The profiling system used for DNA analysis of environmental samples should be documented. The system used should be selected based on the method(s) that would be most commonly used on casework samples generated in the area being monitored. The DNA analysis and acceptance criteria may not be the same as that required for negative controls or consumables’ quality control (QC) batch testing and should be defined.
Interpretation of results and corrective actions
7.8.18 The presence of allelic peaks should be considered at all loci, including amelogenin. Results should be assessed based on the validated interpretation rules applied by the forensic unit undertaking the testing. There should be documented criteria describing the appropriate actions to be taken should human DNA be detected. These criteria may include different actions for different observed levels of contamination (for example, based on the number or heights of the detected allele peaks). Monitoring data should be accessible to the relevant practitioners, together with details of any improvement and corrective actions taken around the period that peaks above background were identified.
7.8.19 A mechanism should also be established to enable feedback to the DNA processing unit where any suspicious (unexpected) or anomalous results have been observed suggesting contamination, so that the DNA unit can investigate and check whether this is the case.
Gross/systemic contamination
7.8.20 In the event of gross/systemic contamination or after maintenance personnel have entered a DNA clean area, a deep clean should be undertaken as detailed in section 7.7.
7.8.21 Where gross/systemic contamination of a particular bench surface or item has occurred or is suspected, the affected area/item should be treated using a validated cleaning regime.
Contaminant reported as a result
7.8.22 In the event that, because of the time required to process the environmental monitoring swabs, a case is reported that has the same profile as the identified contaminant, appropriate action should be taken as required by the circumstances, and stakeholders informed as appropriate (section 9.4).
7.9 Consumables
7.9.1 Consumables are single-use commodities used in the collection, preservation and processing of material for forensic analysis, and are bought and used routinely. These include PPE, tamper evident containers, swabs, and packaging that comes into direct contact with the material for forensic analysis. A consumable can also be equipment used in the collection, processing and safe handling of the material, for example, disposable tweezers or scissors.
7.9.2 Appropriate precautions to minimise the contamination of consumables prior to use include secure storage, restricted access, steps to minimise the chance that the handler causes inadvertent DNA contamination and the risk of DNA being transferred from adjacent items or the storage environment [6].
7.9.3 Consumables should be stored in a dedicated storage area outside of the laboratory. Where consumables are stored within the laboratory for back up purposes, these should be kept in cleanable, sealed container/s.
7.9.4 As stated in the Code (section 103.3.1) consumables used for the recovery of samples for DNA analysis shall be demonstrated to be forensic DNA grade through batch testing and/or using a validated post-production treatment, such as ethylene oxide treatment, to demonstrate clean production standards. Use of DNA consumables compliant with PAS 377 [1] or ISO 18385 [9] negates the need for end-user acceptance batch testing (Code section 103.3.3).
7.9.5 Where batch testing is not required the forensic unit should conduct their own risk assessment to demonstrate confidence, within their Quality Management System that sufficient QA/QC measures are in place. This should include reviewing evidence from the supplier of their compliance with PAS 377 and/or ISO 18385 and the provision of QC data for every batch of consumables, the results for which should be held with the consumables records.
7.9.6 To avoid accidental use of the wrong grade of consumable, forensic DNA grade consumables to be used for the recovery of DNA evidence should be kept separately from non-forensic DNA grade consumables.
7.9.7 Consumables (including PPE) and reagents used shall not be past their expiry date, unless it is verified that they remain fit for purpose beyond that date (Code section 34.2.1).
8. Contamination detection
8.1 Positive and negative controls
8.1.1 The presence of one or more allelic peak(s) in a batch negative control or allelic peak(s) additional to those expected in a positive control is indicative of contamination associated with the DNA laboratory processes. This could have originated from:
a. allele drop-in from very low levels of environmental DNA (unavoidable even in highly controlled DNA-free environments)
b. samples within the same batch or other batches;
c. individuals within the DNA laboratory environment;
d. contaminated consumables; or
e. other sources through secondary transfer mechanisms.
All such contamination should be logged internally by the forensic unit in an appropriate system, to enable contamination to be monitored over time.
8.2 Contamination checks between cases/samples processed (within and between batch comparisons)
8.2.1 Potential instances of cross-contamination should be screened both within and between batches of samples by means of appropriate software comparison programmes, which highlight the degree of similarity (number of shared alleles) between different processed samples.
8.2.2 Within-batch checks should be caried out to identify possible sample-to-sample contamination during DNA processing.
8.2.3 Between-batch checks should be carried out whereby all suitable DNA profiles will be checked against all profiles generated in the same laboratory within an appropriate timeframe, such as the previous three months. This check may identify instances of contamination caused by secondary transfer events, or persistence of DNA on a surface or equipment item that is then transferred to a later sample.
8.2.4 Both of these checks, but in particular the between-batch checks, may well identify matches which are not a result of contamination, but relate to profiles from the same individual analysed on different occasions. These may relate to multiple investigations, or to different samples from the same investigation. The forensic unit will therefore need to investigate all matches to determine those which are likely to represent contamination events. Investigation into these matches may include:
a. Checking batch numbers;
b. Checking processing dates, laboratories, and equipment used; and
c. Reviewing the relevant case circumstances, including dates and geographical locations, to assess the likelihood that DNA from the same person could be found in different samples;
8.2.5 Where casework materials are processed in microtiter plates it is recommended that samples from the same case are not positioned in adjacent wells in order to facilitate detection of potential well to well splash-over.
8.2.6 All instances of cross-contamination shall be logged and monitored by the forensic unit, with investigation of all incidents and corrective actions being undertaken as required.
8.3 Unexpected results
8.3.1 Practitioners reporting DNA results should be suspicious of results that do not fit with case circumstances, including results that are not expected given the material processed or are difficult to explain based on the forensic unit’s validated interpretation guidelines. In some situations it is appropriate to conduct an investigation into the source of the profile including a full assessment of the potential for contamination. Retesting the item may confirm whether the profile is unrelated to the case. All opportunities to discover the cause should be taken as described elsewhere in this guidance.
8.4 Comparison against elimination databases
8.4.1 The Code (section 103.12) requires DNA elimination databases to include:
a. Those involved in the collection/recovery of DNA material, its analysis and the processing environment;
b. Any personnel that have a high-risk of transferring their DNA to items or packaging
c. Any personnel involved in the preparation and assembly of consumable kits and their handling.
8.4.2 The forensic unit may also retain any unexplained/unsourced profiles believed to be the result of contamination.
8.4.3 Prior to submission to the National DNA Database® (NDNAD) and/or reporting of casework results, DNA profiles (as far as possible single-sourced, thereby taking into account the complexity to determine individual contributors from DNA profiles originating from two or more individuals) should be compared against the appropriate DNA elimination database, this could be:
a. the locally held elimination database; and
b. the centrally held contamination elimination database.
9. Management of contamination incidents
9.1.1 If contamination is suspected, the following five-step corrective action process should be undertaken.
9.2 Identify problem
a. Where contamination is suspected the first stage is to investigate and determine the origin of the contaminant profile and the full extent of the problem. This may be achieved by working backwards in a step by step investigative process. Means of contamination detection are set out in section 8.
9.2.2 If the contaminant profile contains too few alleles to allow effective screening, the forensic unit may rework the sample to produce a more informative result by any appropriate means available such as:
a. re-extraction;
b. additional purification steps;
c. dilution of inhibitors;
d. concentrating the extract; and/or
e. application of low template methods; and/or
f. re-examine the item/exhibit.
The use of these troubleshooting procedures should form part of the validated processes used by the forensic unit.
9.3 Determine root cause
9.3.1 The point at which contamination has occurred may be determined by reworking the samples in reverse in a step by step manner – re-electrophoresis, re-polymerase chain reaction (re-PCR), re-extraction. Although alleles under a minimum detection threshold are not reported, these should be considered when performing investigations and corrective actions to assist in determination of the root cause.
9.3.2 Typical root causes may be categorised as follows:
a. Human factors: These include failure of practitioners to comply with standard operating procedures or lack of competence that was either not addressed during training or not identified in competency assessments.
b. System-related: These include a contamination risk not being adequately mitigated during system design and development, which only becomes apparent after validation and implementation, or that the system has materially changed post-validation.
c. A combination of factors including human-related, systems, and others: These include SOPs being insufficiently explicit and therefore open to misinterpretation or omitting information considered to be too obvious to require inclusion, resulting in deviation from the intended procedure by a practitioner(s).
9.3.3 Where human factors are the root cause, it is essential to determine whether this is limited to the work of just one practitioner or more than one, and over what timescale. All potentially affected work should be reviewed, starting at the time of the originally observed contamination incident and working outwards. Dip-testing may be an appropriate means of achieving this in some circumstances. Similarly where there is a systemic issue it is necessary to consider that similar events may have previously gone unnoticed and it may be necessary to review previous work.
9.4 Communication
9.4.1 Case file: Records relating to instances of contamination and any actions agreed either with the commissioning party or internally should be recorded in the relevant case file.
9.4.2 Report: Any contamination detected on a casework sample is disclosable to the Crown Prosecution Service (CPS) and the commissioning party should be notified so the event can be included in any schedule of unused material prepared for the purposes of criminal proceedings. The summary on the disclosure schedule needs to assist the prosecutor in determining whether a report is required, or whether there is a need to disclose.
9.4.3 Stakeholders: Where the occurrence of contamination has consequences beyond the reporting of an individual case, relevant stakeholders shall be informed including:
a. the commissioning party;
b. the Forensic Science Regulator;
c. the Forensic Information Databases Service
d. the CPS; and
e. the UK Accreditation Service (UKAS).
This is required so that additional measures may be considered and taken by the criminal justice system as a whole if required.
9.5 Implement preventative measures
9.5.1 Actions taken to prevent recurrence depend on the root cause.
a. Where a workspace has been affected by a contamination incident and the contamination may still present an issue, items/exhibits should not be processed in the affected workspace until it has been cleaned and the cleaning demonstrated to have been effective through environmental monitoring.
b. Human factor-related issues may require the practitioner(s) in question to complete additional awareness training and competency re-assessment and relevant work should not be carried out until these have been successfully completed.
c. System-related issues may require modification of procedures. These shall be verified as fit for purpose prior to implementation.
d. In all instances, a post-implementation review should be conducted to provide assurance that the preventative measures have been effective.
9.6 Document events
9.6.1 Records of all contamination events, including the investigation and corrective actions taken, should be kept and include:
a. the deficiency;
b. the root cause of the deficiency;
c. the impact on past work;
d. the remedial action taken; and
e. evidence from the post-implementation review that the issue has been resolved.
9.6.2 All corrective actions identified shall be logged and managed within an improvement and corrective action process in accordance with the requirements of ISO/IEC 17025:2017.
10. Management oversight and continuous improvement
(ISO/IEC 17025:2017 sec. 8)
10.1.1 There should be governance and oversight of contamination avoidance, monitoring and detection by the forensic science provider’s senior management. This should include a technical authority with responsibility for assessment and review of contamination, including responsibility for escalating contamination issues to senior management where required.
10.1.2 Maintaining a log of contamination events and periodically reviewing these to identify trends and the potential for further contamination control measures should be undertaken by the management technical authority and be part of an overall continuous improvement process.
10.1.3 There should be good communication with practitioners and practitioners should be able to demonstrate ownership of contamination issues. Improvement at the team/unit level should also be encouraged.
10.1.4 Periodic management reviews of contamination should be undertaken by the forensic unit on a six-monthly basis as a minimum. These reviews should monitor all serious contamination events and provide a summary of low-level contamination (low-level contamination can also include minor components in mixture profiles) observed within DNA processes including:
a. contamination observed;
b. testing of consumables where this is undertaken;
c. contamination rates within batch controls;
d. batch re-run and re-extraction rates due to contamination; and
e. environmental monitoring results.
10.1.5 To enable overall trends to be monitored, contamination reviews should assess contamination trends within the forensic unit and be made available to:
a. the Forensic Science Regulator;
b. the UK Accreditation Service; and the
c. Forensic Information Databases Service .
10.1.6 After implementation of new methods/systems, post-implementation reviews should be carried out, including monitoring of any change in contamination rates.
11. Review
11.1.1 The published guidance will form part of the review cycle as determined by the Forensic Science Regulator.
11.1.2 The Forensic Science Regulator welcomes views on this guidance. Please send any comments to the address as set out at the following web page: www.gov.uk/government/organisations/forensic-science-regulator or send them to the following email address: FSREnquiries@forensicscienceregulator.gov.uk.
12. Acknowledgements
12.1.1 This guidance has been adapted from the previous, non-statutory guidance (FSR-G-208, The Control and Avoidance of Contamination in Laboratory Activities involving DNA Evidence Recovery Analysis) and reviewed by the Regulator’s DNA specialist group.
13. References
-
British Standards Institute, “PAS 377 - Consumables used in the collection, preservation and processing of material for forensic analysis – Product, manufacturing and forensic kit assembly – Specification, 2023. (Online). (ccessed 26 7 2023).
-
International Organization for Standardization, BS ISO 18385:2016 Minimising the risk of human DNA contamination in products used to collect, store and analyse biological material for forensic purposes - Requirements, ISO, 2016.
-
K. Ballantyne, R. Salemi, F. Guarino, J. Pearson, D. Garlepp, S. Fowler and R. van Oorschot, “ DNA contamination minimisation – finding an effective cleaning method,” Australian Journal of Forensic Sciences, vol. 47, no. 4, pp. 428-39, 2015
-
British Standards Institute, “BS EN ISO 14644 - Cleanrooms and associated controlled environments”, British Standards Institute, 2015. (Online). (Accessed 25 7 2023).
-
ISO, Lighting of work places - Part 1: Indoor, 2002.
-
Health and Safety Executive, Lighting at work, Suffolk: HSE, 1997.
-
A. E. Fonneløp, T. Egeland and P. Gill, “‘Secondary and subsequent DNA transfer during criminal investigation’,” Forensic Science International: Genetics, vol. 17, p. 155–162, 2015.
-
J. A. Bright, S. Cockerton, S. Harbison, A. Russell, O. Samson and K. Stevenson, “‘The effect of cleaning agents on the ability to obtain DNA profiles using the Identifiler™ and PowerPlex® Y multiplex kits,” J. Forensic Sci., vol. 56, no. 1, p. 181–85, 2011. |
14. Further reading
Al-Snan, N.R. and Alraimi, N.M. (2022) ‘Comparison between various DNA sterilization procedures applied in forensic analysis’. Egyptian Journal of Forensic Sciences 12:5.
Ballantyne, K.N., Poy, A.L., van Oorschot, R.A.H., (2013) ‘Environmental DNA monitoring: beware of the transition to more sensitive typing methodologies’, Aust. J. Forensic Sci. 45 (3) 323–340.
Basset, P., Castella, V. (2018) ‘Lessons from a study of DNA contaminations from police services and forensic laboratories in Switzerland’, Forensic Sci. Int. Genet. 33 147–154.
Davies, C., Thomson, J. and Kennedy, F. (2015) ‘Assessing primary, secondary and tertiary DNA transfer using the Promega ESI-17 Fast PCR chemistry’, Forensic Science International: Genetics Supplement Series, 5, pp e55–e57.
Gill, P. (2019) ‘DNA evidence and miscarriages of justice’. Forensic Sci. Int., 294, e1–e3.
Goray, M., Fowler, S., Szkuta, B. and van Oorschot, R.A.H. (2016) ‘Shedder status—An analysis of self and non-self DNA in multiple handprints deposited by the same individuals over time’, Forensic Science International: Genetics, vol. 23, pp 190–196.
Hashiyada, M., Nakanishi, H., Asogawa, M., Akane, A., Saito, K., Miyachi, H., Osawa, M. (2022) ‘Removal effect of DNA contamination by hydrogen peroxide plasma compared to ethylene-oxide gas’. Legal Medicine 54.
IFSA (2021) ‘Minimum Requirements for DNA Collection, Analysis and Interpretation - A document for emerging laboratories’. Version 2. (Accessed 02/06/23).
Kampmann, M.-L., Børsting, C., Morling, N. (2017) ‘Decrease DNA contamination in the laboratories’, Forensic Sci. Int. Genet. Suppl. Ser. 6 e577– e578.
Kampmann, M-L., Simonsen, B.T., Børsting, C. (2022) ‘Test of chlorine wipes for efficient removal of DNA from forensic genetics laboratories’. Forensic Sci. Int. Genet. Suppl. Ser.
Lapointe, M., Rogic, A., Bourgoin, S., Jolicoeur, C. and Séguin, D. (2015) ‘Leading-edge forensic DNA analyses and the necessity of including crime scene investigators, police officers and technicians in a DNA elimination database’, Forensic Science International: Genetics, vol.19, pp 50–55.
Lehmann, V. J., Mitchell, R. J., Ballantyne, K. N. and van Oorschot, R. A. H. (2015) ‘Following the transfer of DNA: How does the presence of background DNA affect the transfer and detection of a target source of DNA?’, Forensic Science International: Genetics, vol. 19, pp 68–75.
Liebers, V., Bachman, D., Franke, G., Freundt, S., Stubel, H., Duser, M., Kendzia, B., Bockler, M., Brunning, T. and Raulf, M. (2015) ‘Determination of ATP Activity as a Useful Tool for Monitoring Microbial Load in Aqueous Humidifier Samples’, International Journal of Hygiene and Environmental Health, vol. 218, issue 2, pp 246–253.
Manoli, P., Antoniou, A., Bashiardes, E., Xenophontos, S., Photiades,
M., Stribley, V., Mylona, M., Demetriou, C. and Cariolou, M. A. (2016) ‘Sex- specific age association with primary DNA transfer’, International Journal of Legal Medicine, vol.130 (1), pp 103–112.
Meakin, G. and Jamieson, A. (2013) ‘DNA transfer: Review and implications for casework’, Forensic Science International: Genetics, vol. 7, issue 4, pp 434–43.
Mercer, C., Henry, J., Taylor, D., Linacre, A. (2022) What’s on the bag? The DNA composition of evidence bags pre- and post-exhibit examination. Forensic Science International: Genetics 57
National Institute of Justice Forensic Technology Center of Excellence, (2011) Comparison Study of Disinfectants for Decontamination, Award No. 2010-DN- BX-K210. (Accessed 18/08/2020).
National Institute of Justice Forensic Technologies Center of Excellence, (2012) Swab Collection Study, Award No. 2010-DN-BX-K210. (Accessed 18/08/2020).
Nilsson, M., De Maeyer, H., Allen, M. (2022) ‘Evaluation of Different Cleaning Strategies for Removal of Contaminating DNA Molecules’. Genes, 13, 162.
Pickrahn, I., Kreindl, G., Müller, E., Dunkelmann, B., Zahrer, W., Cemper- Kiesslich, J.; Neuhuber, F. (2017) ‘Contamination incidents in the pre-analytical phase of forensic DNA analysis in Austria - statistics of 17 years’, Forensic Sci. Int. Genet. 3112–18.
Quinones, I. and Daniel, B. (2012) ‘Cell free DNA as a component of forensic evidence recovered from touched surfaces’, Forensic Science International: Genetics, vol. 6, no. 1, pp 26–30.
Stella, C.J., Meakin, G.E., van Oorschot, R.A.H. (2022) ‘DNA transfer in packaging: Attention required’. Forensic Sci. Int. Genet. Suppl. Ser.
Sweet, D., Lorente, M., Lorente, J. A., Valenzuela, A. and Villanueva, E. (1997) ‘An improved method to recover saliva from human skin: The double swab technique’, Journal of Forensic Science, 42 (2), pp 320–22.
Szkuta, B., Harvey, M. L., Ballantyne, K. N. and van Oorschot, R. A. H. (2015) ‘DNA transfer by examination tools – a risk for forensic casework?’, Forensic Science International: Genetics, vol. 16, pp 246–54.
Taylor, D., Bright, J. A., McGoven, C., Hefford, C., Kalafut, T. and Buckleton, J. (2016) ‘Validating multiplexes for use in conjunction with modern interpretation strategies’, Forensic Science International: Genetics, vol. 20, pp 6–19.
Taylor, D., Abarno, D., Rowe, E. and Rask-Nielsen, L. (2016) ‘Observations of DNA transfer within an operational Forensic Biology Laboratory’, Forensic Science International: Genetics, vol. 23, pp 33–49 .
Toothman, M. H., Kester, K. M., Champagne, J., Cruz, D. T. W., Street, S. and Brown, B. L. (2008) ‘Characterization of human DNA in environmental samples’, Forensic Science International: Genetics, vol. 178, issue 1, pp 7–15.
Vandewoestyne, M., Van Hoofstat, D., Franssen, A., Van Nieuwerburgh, F. and Deforce, D. (2013) ‘Presence and potential of cell free DNA in different types of forensic samples’, Forensic Science International: Genetics, vol. 7, issue 2, pp 316–20.
van Oorschot, R. A. H., Ballantyne, K. N. and Mitchell, R. J. (2010) ‘Forensic trace DNA: a review’, Investigative Genetics 1:14. Accessed 18/08/2020.
van Oorschot, R. A. H., Glavich, G. and Mitchell, R. J. (2014) ‘Persistence of DNA deposited by the original user on objects after subsequent use by a second person’, Forensic Science International: Genetics, vol. 8, issue 1, pp 219–25.
van Oorschot, R.A.H., Found, B., Ballantyne, K.N. (2015) Considerations Relating to the Components of a Laboratory DNA Contamination Minimisation Monitoring (DCMM) Program, Forensic Science Policy & Management: An International Journal Volume 6, Issue 3-4, p 91-105.
van Oorschot, R.A.H., Meakin, G.E., Kokshoorn, B., Goray, M., Szkuta, B. (2021) ‘DNA Transfer in Forensic Science: Recent Progress towards Meeting Challenges’. Genes 12, 1766.
15. Abbreviations And Acronyms
- BS: British Standard
- CJS: Criminal Justice System
- CPS: Crown Prosecution Service
- DNA: Deoxyribonucleic acid
- FSR: Forensic Science Regulator
- HEPA: high efficiency particulate air
- HSE: Health and Safety Executive
- IEC: International Electrotechnical Commission
- ISO: International Organisation for Standardization
- IT: Information Technology
- NDNAD: National DNA Database®
- PAS: Publicly Available Specification
- PCR: Polymerase chain reaction
- PPE: Personal protective equipment
- QA: Quality assurance
- QC: Quality control
- SED: Staff elimination database
- SOP: Standard operating procedure
- STR: Short tandem repeat
16. Glossary
Allelic peak
A peak that falls within an allelic window, has well defined allele morphology and a peak height greater than the defined limit of detection of the laboratory.
Crime sample
An item/exhibit or sub-item/exhibit recovered and believed to provide evidence to investigate or prosecute a criminal offence, i.e. crime-related.
DNA contamination
The undesirable introduction of DNA, or biological material containing DNA, to an item/exhibit or subsample derived from an exhibit, which is to be examined/analysed. Gross contamination is where a partial or complete DNA profile from a single person (these alleles are ‘dependent’) is obtained as a result of a contamination event. Systemic contamination is where there is general, universal contamination across a batch or between batches of test results.
Laboratory
Any area in which the packaging of an exhibit is opened, or items for DNA analysis are processed, including drying rooms.
Unsourced contaminant
A DNA profile identified as a contaminant but that following all relevant elimination database checks the source has not been identified. No template (negative) controls and quality control batch tests are considered as having originated from the manufacturing supply chain, historically most have beenfound to come from manufacturing staff.
Published by:
The Forensic Science Regulator
23 Stephenson Street
Birmingham B2 4BJ.
https://www.gov.uk/government/organisations/forensic-science-regulator

