MAVIS edition 102
Published 26 April 2017
1. News
1.1 VMD Pharmaceutical Industry Information Event on 23 June 2017
The VMD will be holding an Information Event for the pharmaceutical industry on Friday 23 June 2017. We are currently developing the schedule for the day and we are inviting your suggestions on areas you would like to see covered.
The event usually starts around 10am until 3pm. Timings will be confirmed once we have the final agenda.
If you would like to attend, please register your interest to Natalie Burge n.burge@vmd.defra.gsi.gov.uk by Wednesday 31 May 2017.
1.2 Restrictions on Schedule 6 (Exemptions for small pet animals) active substances
The VMD has recently reviewed the approved actives on the exemption for small pet animals (also known as the Small Animal Exemption Scheme) which may currently be marketed for use as anaesthetics in fish. The indications for these products are now restricted to mild sedation and euthanasia of diseased fish only.
The basis of the change is the VMD’s reviewed position regarding interpretation of the Veterinary Surgeons Act and conclusion that anaesthesia should be considered an act of veterinary surgery.
The table listing the approved actives has been amended to include the warning; “Products containing [approved active] may be indicated for mild sedation and also for euthanasia of diseased fish. Indications for anaesthesia are not permitted in products marketed under this exemption”.
The list of all actives approved under the exemption and further guidance can be found on GOV.UK.
2. Licensing
2.1 Guidance for applicants regarding applications to be submitted under Article 13
Following a number of queries the Co-ordination Group for Mutual Recognition and Decentralised Procedures (veterinary) (CMDv) discussed whether products which have at least some local action such as topical skin/eye/ear products, spot-on ectoparasiticides, intramammary products and products acting locally within the gastrointestinal tract, should be submitted either under Article 13(1) (generic) or Article 13(3) (so called ‘hybrid’) of Directive 2001/82/EC, as amended by 2004/28/EC.
The conclusion was that the Reference Member State (RMS) for the procedure should decide and provide advice to the applicant as to the appropriate Article to use.
The UK position is that products that do not satisfy the definition of a ‘generic’ product as defined in Article 13(2) of the Directive, i.e. where bioavailability cannot be demonstrated due to a lack of measureable plasma levels and are therefore not captured by the Committee for Medicinal Products for Veterinary Use (CVMP) guideline for the conduct of bioequivalence studies, should be submitted via Article 13(3), as also reflected in Volume 6B of the Notice to Applicants.
For locally acting products not currently covered by guidance, such as ear and eye preparations or products acting within the gastrointestinal tract, the VMD will consider extrapolation of the principles behind section 7.1(d) of the bioequivalence guideline, where scientifically justified.
As for all matters regarding authorisation of veterinary medicines, pharmaceutical companies are invited to contact the VMD or arrange a meeting if there is any doubt over which Article should be used, and whether a waiver could be applicable.
2.2 Marketing Authorisations
We no longer publish information about varied Marketing Authorisations (MAs). Details of clinically significant variations are published in the Veterinary Record.
Information on newly authorised and expired MAs is available in the Product Information Database (PID). You can:
- go to the homepage and select the ‘Recently Authorised MAs’ tab for a list of MAs authorised in the previous 6 months
- go to the Expired tab to see a list of expired MAs
2.3 Quarterly reporting against VMD Published Standards for 2016-2017 licensing work
This report is published on a monthly basis under Our Statistics on GOV.UK.
For further information contact Natalie Shilling n.shilling@vmd.defra.gsi.gov.uk and put ‘MAVIS’ in the subject line.
2.4 Top ten imported veterinary medicines quarterly report from 1 January to 31 March 2017
The VMD provides a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
| Product | Active Ingredient | No. of Certificates Issued |
|---|---|---|
| Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,665 |
| Filavac VHD K C+V | Rabbit Haemorrhagic Disease (Inactivated) | 477 |
| Vet-Goid | Allergens | 312 |
| Nobivac Myxo-RHD, lyophilisat et solvant pour suspension injectable pour lapins | Myxoma vectored RHD virus (live) | 237 |
| Spectrum Hyposensitisation Vaccine - Injectable Solution | Allergen | 220 |
| Botulism Vaccine | Clostridium botulinum type C & type D toxoid | 182 |
| Greer Allergenic Extract Patient Prescription | Allergens | 151 |
| Pneumabort-K +1b | Equine Rhinopneumonitis Virus | 148 |
| Artuvetrin® Test, injection fluid for intracutaneous use in dogs | Allergens | 110 |
| Salmopast | Pasteurella Multocida Mannheimia haemolytica Salmonella Dublin Salmonella Typhimurium | 106 |
For further information contact Renee Sheehan r.sheehan@vmd.defra.gsi.gov.uk
3. Inspections and investigations
Breaches of the VMR are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice Inspection Team (GMPIT)
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
Guidance is available on obtaining a UK manufacturing authorisation (ManA) for an authorised veterinary medicine or an authorisation for an extemporaneous product (special) (ManSA) on GOV.UK.
Guidance is also available on GOV.UK on obtaining a manufacturing authorisation for an equine stem cell centre (ESCCA), a non-food animal blood bank (NFABBA) or an autogenous vaccine (AVA).
3.2 GMPIT inspection deficiency classification
GMP inspections can result in the citation of deficiencies. The number and severity of deficiencies influences the required re-inspection frequency.
Critical Deficiency:
- A deficiency which has produced, or leads to a significant risk of producing either a product which is harmful to the human or veterinary patient or a product which could result in a harmful residue in a food producing animal.
Major Deficiency:
A non-critical deficiency:
- which has produced or may produce a product, which does not comply with its marketing authorisation
- or which indicates a major deviation from EU Good Manufacturing Practice
- or (within EU) which indicates a major deviation from the terms of the manufacturing authorisation
- or which indicates a failure to carry out satisfactory procedures for release of batches or (within EU) a failure of the Qualified Person to fulfil his legal duties
- or a combination of several “other” deficiencies, none of which on their own may be major, but which may together represent a major deficiency and should be explained and reported as such
Other Deficiency:
- A deficiency, which cannot be classified as either critical or major, but which indicates a departure from good manufacturing practice.
3.3 GMPIT inspection deficiency findings 1 April 2016 to 31 March 2017
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period.
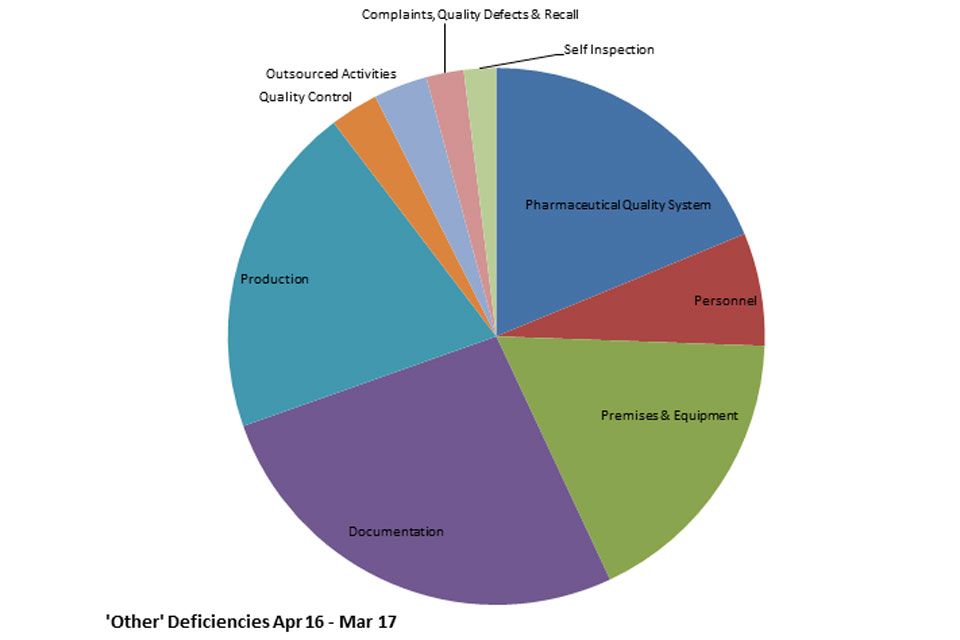
GMP Inspections Other deficiencies April 2016 to March 2017
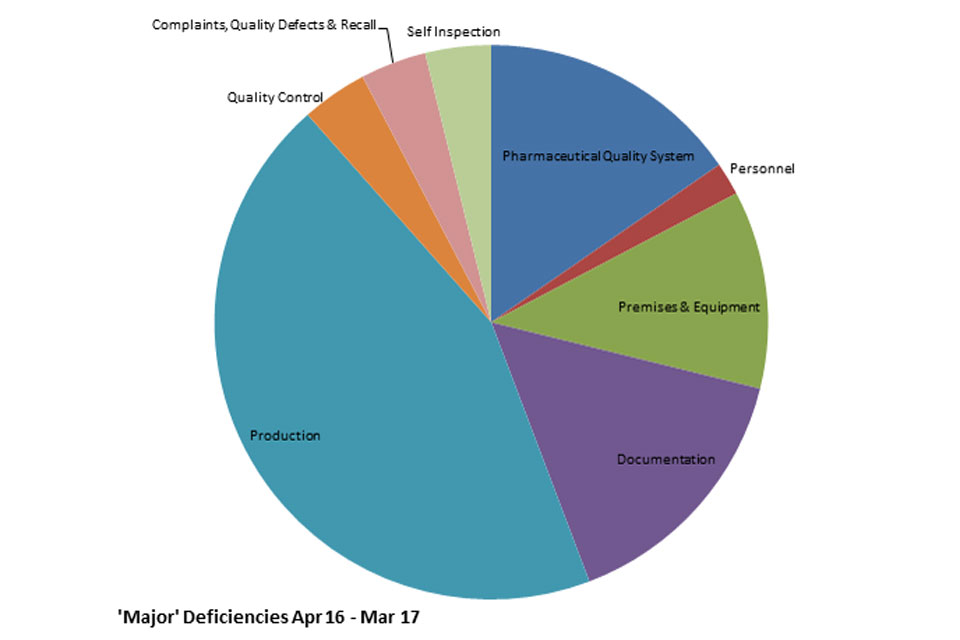
GMP Inspections Major deficiencies April 2016 to March 2017
3.4 The Inspections and Investigations Team (IIT)
Guidance is available for:
- manufacturers and distributors of animal feedingstuffs containing veterinary medicines and/or coccidiostats (referred to in the VMR as specified feed additives – SFAs)
- veterinary-only wholesale dealers to assess compliance against GDP
- premises for the retail supply of POM-VPS and NFA-VPS medicines by suitably qualified persons (SQPs)
- veterinary practice premises (VPPs), other than those accredited by the Royal College of Veterinary Surgeons (RCVS) under its voluntary Practice Standards Scheme (PSS).
3.5 Change to Wholesale Dealer Authorisation (WDA) application and variation forms
We have removed the ‘Product Classes’ section from the Wholesale Dealer Authorisation (WDA) Application Form and Variation Form. Therefore, applicants completing those forms will no longer have to specify the product classes (pharmaceutical forms) that they wish to wholesale. We have made this change in response to a number of applicants telling us that it is difficult to specify the product classes that they may wish to supply in the future. Having reviewed the requirement for pharmaceutical forms to be specified on the application and variation forms, we have decided it is unnecessary. This change will also mean that wholesale dealers will not have to submit a variation if they want to add or remove a product class from their range of products.
Current Wholesale Dealer Authorisation holders will receive reissued documents following an inspection or a variation.
This change only applies to veterinary-only wholesale dealer authorisations issued by the VMD.
For further information contact Justin Murphy inspections@vmd.defra.gsi.gov.uk
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 December 2016 to 28 February 2017, the VMD received 1,526 animal suspected adverse event reports. Of the 2,204[footnote 1] products involved in these reports, 2,077 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 1,934 | Prescription Only Medicine Veterinarian (POM V) |
| 96 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 27 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 20 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 127 products were:
| Amount | Type |
|---|---|
| 43 | authorised human medicines |
| 20 | medicine used in a trial under an Animal Test Certificate |
| 14 | veterinary products without any medicinal claim |
| 9 | authorised medicines imported from other countries |
| 7 | medicines sold under the exemption for small pet animals |
| 2 | specially formulated veterinary medicines |
| 1 | biocide |
| 31 | unidentified products |
During this period 40 reports of human suspected adverse reactions were received. Of the 43[footnote 1] products involved in these reports, 42 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 35 | Prescription Only Medicine Veterinarian (POM V) |
| 4 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 1 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 2 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining product was a specially formulated veterinary medicine.
No environmental incident reports were received during this period.
For further information contact Roy Savory r.savory@vmd.defra.gsi.gov.uk
5. Enforcement
A list of prosecutions and notices involving illegal activity with veterinary medicines in the last year can be found on GOV.UK.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 UK Antimicrobial Resistance (AMR) Strategy
The most recent meeting of the High Level Steering Group (HLSG) for the AMR Strategy took place on 17 February 2017. Activities to deliver the aims of the Strategy are being implemented in line with the guidance of the HLSG.
6.2 Sales Data Report and Antibiotic Resistance Surveillance Report
Collation of the 2016 antimicrobial sales data is underway and Marketing Authorisation holders have been invited to submit sales figures for 2016. The full 2015 report, highlights and previous years reports can be found on GOV.UK.
6.3 Defra Antimicrobial Resistance Co-ordination (DARC)
The DARC group met on 21 September 2016 to discuss recent trends in antibiotic resistance in bacteria of importance to human and animal health. The group also discussed investments and initiatives relating to AMR, headline antibiotic sales and resistance findings, the Independent Review on AMR, recycled manure solids for use as a bedding material and the One Health Report. The DARC group also met on 9 February 2017; topical discussions focused on the existing evidence and current data gaps for antimicrobial resistance in companion animals. A summary of this meeting will be published on GOV.UK shortly. The next DARC meeting is scheduled for 29 June 2017.
6.4 European Surveillance of Antimicrobial Consumption (ESVAC)
The ESVAC annual network meeting was held at the European Medicines Agency (EMA) on 2 and 3 March 2017. Preliminary analysis of 2015 antibiotic sales data across the EU was presented and discussed, as well as the draft ESVAC Vision and Strategy 2016-2020.
ESVAC recently published draft guidance on the collection of antimicrobial use data by species and is available for public consultation over the next 6 months until 24 September 2017. It can be read together with a Question and Answer document on the guidance.
For further information contact Stacey Brown s.brown@vmd.defra.gsi.gov.uk
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The January VPC meeting was cancelled. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
For further information contact Lea Stott l.stott@vmd.defra.gsi.gov.uk
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2016 and full details of UK results, together with information on any action taken, can be found on GOV.UK.
For further information contact Sandra Russell s.russell@vmd.defra.gsi.gov.uk
9. Staff changes
The following staff changes took place during this quarter.
New staff:
- Anna Pracel joined the Good Manufacturing Practice Inspections team and Jennifer Underwood joined the Inspection and Investigations team
- Luke Wakefield joined as Head of the IT team
- Bindu Menon joined the Committee and Office Support team
- Matthew Hulett joined the Licensing Administration team
- Miguel Escribano joined the Pharmaceuticals and Feed Additives team
Departing staff:
- Suzanne Eckford was seconded to the Food and Agriculture Organisation of the United Nations
- Sue Rawlinson was seconded to the Home Office
- Neil Paterson and David Webb retired
- Carole Lutley resigned
Movements within the VMD:
- Callum Harris moved to the Pharmaceuticals and Feed Additives team
- Rutendo Manyarara has been temporarily promoted to the Head of the Pharmaceuticals and Feed Additives team and Claire Stratford and John Mitchell were also temporarily promoted within the team
- Andy Parker and Debbie Austin were promoted within the Licensing Administration team
- Carol Siwicka transferred to the Communications team
10. Organogram as at 1 April 2017
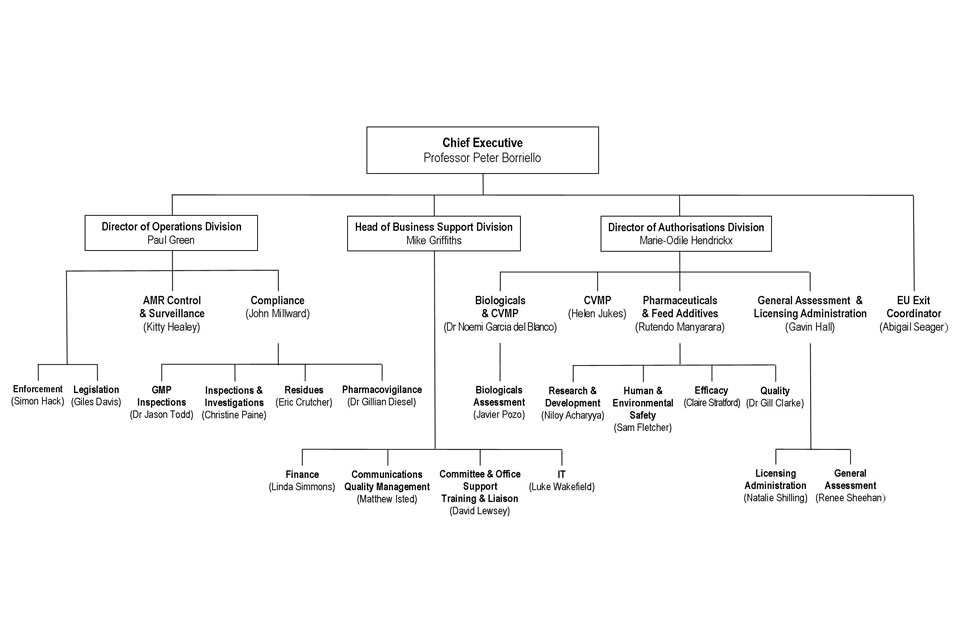
VMD Organogram April 2017
