HAIRS risk assessment: Crimean-Congo haemorrhagic fever
Updated 28 March 2024
About the Human Animal Infections and Risk Surveillance group
This document was prepared by the UK Health Security Agency (UKHSA) on behalf of the joint Human Animal Infections and Risk Surveillance (HAIRS) group.
HAIRS is a multi-agency cross-government horizon scanning and risk assessment group, which acts as a forum to identify and discuss infections with potential for interspecies transfer (particularly zoonotic infections). Its work cuts across several organisations, including:
-
UKHSA
-
Department for Environment, Food and Rural Affairs (Defra)
-
Department of Health and Social Care (DHSC)
-
Animal and Plant Health Agency (APHA)
-
Food Standards Agency (FSA)
-
Public Health Wales (PHW)
-
Public Health Scotland (PHS)
-
Department of Agriculture, Environment and Rural Affairs for Northern Ireland (DAERA)
-
Welsh Government
-
Scottish Government
-
Public Health Agency of Northern Ireland
-
Department of Agriculture, Food and the Marine, Republic of Ireland
-
Health Service Executive, Republic of Ireland
-
Infrastructure, Housing and Environment, Government of Jersey
-
Isle of Man Government
-
States Veterinary Officer, Bailiwick of Guernsey
Information on the risk assessment processes used by the HAIRS group can be found on GOV.UK.
Version control
Date of this assessment: March 2024
Date of initial risk assessments: V1.0 April 2017; V2.0 December 2018; V3.0 April 2021
Version: 4.0
Reason for the assessment: Updated to reflect the geographical expansion of CCHF virus (for example, detections in ticks in the south of France) and to incorporate the latest epidemiology and scientific literature.
Completed by: HAIRS members and external experts
Non-HAIRS members consulted:
-
Dr. Kayleigh Hansford, UKHSA’s Medical Entomology and Zoonoses Ecology Team
-
Dr. David Mesher, Lead Epidemiologist, UKHSA’s Emerging Infections and Zoonoses Team
-
Dr. Sherine Thomas, Consultant in UKHSA’s Emerging Infections and Zoonoses Team
Summary
Crimean-Congo haemorrhagic fever (CCHF) is a tick-borne disease which cycles between a wide range of domestic and wild animals, with humans infected via tick bites or contact with infected blood and tissues. It is not present in the United Kingdom (UK), nor are there any identified established populations of Hyalomma ticks, the principal vectors of CCHF virus (CCHFV). However, there are multiple routes for the introduction of the tick into the UK, including through the movement of migratory birds.
In 2016, Spanish authorities reported the first autochthonous clinical cases of CCHF in Spain and in Western Europe, with a retrospective analyses identifying human cases dating back to 2013. Ticks in Spain had first been found to carry CCHFV in 2010, therefore the occurrence of CCHF human cases there was not unexpected. Since 2013, and as of February 2024, 12 human cases of CCHFV infection have been reported in Spain. Additionally, in October 2023, French officials reported the detection of CCHFV in Hyalomma marginatum ticks collected from cattle in the eastern Pyrénées. These were the first detections of CCHFV in ticks in France.
In 2018, 2 Hyalomma tick species were detected in the UK. An adult male H. rufipes tick was detected in September 2018 on a horse with no history of travel. This suggests possible moulting for the first time of an imported H. rufipes nymph (via a migratory bird) to an adult, despite UK climate constraints previously considered to be a limiting factor for tick development. Additionally, an adult male H. marginatum tick (not feeding) was found on a human at a bird reserve in Norfolk in 2018. This is likely the result of a moulted nymph and the first evidence of H. marginatum moulting in the UK. This concurs with other reports in northern Europe during 2018 of Hyalomma ticks being found outside their endemic range during an unusually warm summer period. In July 2022, an adult male H. marginatium tick was found on a horse in the South-East of England, but this could not be confirmed as UK acquired.
As of February 2024, 2 travel associated human cases of CCHF have been reported in the UK. No locally acquired human cases have been reported to date, nor has CCHFV been found in any potential tick vector species found within the UK.
Assessment of the risk of infection in the UK:
Probability
Currently, the probability of infection is considered Very Low for the UK population.
Impact
The impact on the UK population is considered Very Low to Moderate.
Level of confidence in assessment of risk
Good.
The quality of evidence used in this assessment is considered mostly good, and thus the confidence of the risk assessment output is good (high quality evidence from multiple reliable sources and verified, expert opinion concurs).
Actions and/or recommendations
Vector surveillance:
-
continue close monitoring of the situation in Europe for further geographical expansion of CCHF tick vector species
-
continue monitoring for new evidence as to the presence of Hyalomma species in the UK through existing UK tick surveillance schemes
-
consider broader surveillance for Hyalomma species, including targeted tick surveillance through occupational groups (for example, equine veterinary professionals)
-
raise professional and public awareness to tick surveillance schemes within the UK
-
upon detection of Hyalomma ticks in the UK, ensure data is shared between agencies and HAIRS is informed for joint monitoring and interpretation
-
ensure all submissions of Hyalomma ticks to tick surveillance schemes are followed up by agencies to assess whether they are locally acquired, or travel associated
-
Hyalomma ticks identified in the UK should be tested for CCHFV
-
consider inter stakeholder contingency planning for invasive tick species, such as controlling tick infestations on animals or in stables and barns
For animal health professionals:
-
ticks identified on animals should be submitted to tick surveillance schemes
-
raise awareness with animal keepers of potential for tick establishment where animals and birds co-exist (for example, in stables)
For public health professionals:
-
continue to monitor the situation in European countries for increasing reports of autochthonous cases or changes in pathogenicity for human infections
-
raise awareness on tick avoidance measures for the public, particularly travellers to known affected areas
-
clinicians should be made aware of the clinical features of CCHF and consider it early in travellers with suspect symptoms who have visited endemic areas and taken part in higher risk activities (for example, outdoor activities, animal slaughter)
-
ensure appropriate clinical assessment and public health management protocols are available to manage a human case of CCHF in the UK
Step 1. Assessment of the probability of infection in the UK human population
This section of the assessment examines the likelihood of an infectious threat causing infection in the UK human population. Where a new agent is identified there may be insufficient information to carry out a risk assessment and this should be clearly documented. Please read in conjunction with the Probability algorithm.
Is this a recognised human disease?
Outcome
Yes.
Quality of evidence
Good.
CCHF was first described in the Crimea in 1944, where an outbreak of an acute febrile illness with a high incidence of shock and bleeding occurred among soldiers and agricultural workers. In 1969, it was recognised that the virus causing this disease was identical to a virus that had been isolated from a child in the Congo in 1956 (1). Humans (and possibly non-human primates (2) are the only animal species known to manifest severe clinical CCHF disease (3, 4).
CCHF virus (CCHFV) is a member of the family Nairoviridae, in the genus Orthonairovirus (5). CCHFV exhibits great sequence diversity. From analysis of its S, L and M segments, phylogenetic trees have been constructed containing lineages I to VI. CCHFV is thought to have originated in Africa between 1,000 and 5,000 years ago, although a strain (Ap92) found in Greece is considered an ancient lineage. The virus was introduced to Central and South Asia in the Middle Ages, with spread into Europe considered a more recent event, likely via a single introduction into central Russia. Westward spread has since taken place, with further geographical expansion considered likely (6, 7).
CCHF has a widespread geographical distribution. It is considered endemic in countries in Africa, the Balkans, the Middle East, western and south-central Asia. It is estimated that globally between 10,000 and 15,000 human infections, including approximately 500 fatalities, occur annually (8). The distribution of human cases corresponds to the geographical range of established Hyalomma tick species (3), notably H. marginatum and H. lusitanicum, which are considered the primary vectors of CCHFV (4).
Globally, there have been case reports, virological or serological evidence of human infection in at least 55 countries (3, 9). In the European Region and its neighbouring countries, locally acquired human cases and/or outbreaks have been reported from Albania, Bulgaria, Georgia, Greece, Kosovo, Russia, Spain, Turkey and Ukraine (8, 10). Spain officially reported its first autochthonous case in August 2016, the first in Western Europe, following their first detection of CCHFV infected ticks in 2010 (11 to 13). However, retrospective analyses identified human cases in western Spain dating back to summer 2013 (14). As of January 2024, a total of 12 confirmed cases have been officially reported from Spain, including one case of nosocomial transmission in 2016, and 4 fatalities (one each in 2016, 2018, 2020 and 2022) (10). A CCHFV seroprevalence study carried out between 2017 and 2018 in Castile-León, a Spanish region where CCHF cases had been reported, found evidence of past exposure to CCHFV of between 0.58% and 1.16% in healthy blood donors (results varied dependent on assay used) (15). CCHFV genotypes Africa III and Europe V have been detected both in human cases (16) and in different tick species (H. lusitanicum and Dermacentor marginatus) collected from various wild ungulate hosts (red and fallow deer; Eurasian wild boar) across south-west Spain (17), showing the wide spatial presence of CCHFV in this region. Authorities state there is a moderate likelihood of future cases in known CCHF risk areas, although the impact would be low given the expected small number of cases and availability of adequate means of isolation and control of cases (18).
At the end of October 2023, French officials reported the detection of CCHFV in H. marginatum ticks collected from cattle in the eastern Pyrénées as part of an active surveillance project on equestrian and cattle premises in the region (19). Whilst H. marginatum have established populations in southern France, this was the first time the presence of the virus in tick populations had been confirmed in the country. This geographical expansion of CCHFV is consistent with model predictions by Messina and Wint (20), who concluded that environmental suitability for the disease likely extends as far as north-west France and central Europe, compared to 2015 model predictions (20, 21). As of January 2024, no autochthonous human cases have ever been reported in France.
CCHF disease has varying manifestations from asymptomatic or subclinical infection through to fulminant haemorrhage. The incubation period is usually less than 14 days (typically 3-7 days), although this duration varies depending on factors including viral dose and route of exposure; it is often shorter following nosocomial infection (8). The onset of symptoms is sudden, with fever, severe headache, dizziness, photophobia, malaise, myalgia and back pain reported. Sore throat, nausea, vomiting and diarrhoea may also feature. Hepatomegaly and lassitude can develop after 2 to 4 days (1, 22, 23). The haemorrhagic forms of the disease are more diverse than other viral haemorrhagic fevers, starting with a petechial rash, which usually appears in the first week of illness and progressing through extensive bruising and bleeding under the skin, to excessive bleeding in the second week of illness in those with severe infection (23). Cerebral haemorrhage has also been described (24). In fatal cases, death occurs from haemorrhage, multi-organ failure and shock usually between days 5 and 14 of illness (1). Reported overall case fatality rates have varied from 5% to more than 40%, though this disparity is likely skewed by small sample sizes and failure to detect and report less severe cases (1). It is possible that subclinical infections represent a substantial proportion of cases; thus resulting in under ascertainment of the true number of cases (4).
CCHFV is maintained in several ixodid (hard) tick species and these are responsible for spreading the virus to a wide range of wild and domestic animals. Illness has not been reported in these mammals, but there is evidence of transient viraemia for up to 15 days (25). Some mammals, and probably some avian species, act as amplifying hosts that can subsequently infect ticks feeding on them (1, 26). Human disease from an animal source can occur following exposure to blood and/or body fluids of infected animals (particularly livestock) and via the bite of an infected tick (22). In areas where CCHFV is known to circulate, a higher exposure risk may therefore exist for people participating in outdoor activities (for example farmers, game hunters, forestry workers and hikers) and those who have prolonged direct exposure to livestock and animal products (for example shepherds, farmers, butchers, slaughterhouse workers and veterinarians) (4).
Contaminated meat is generally not considered a potential source of exposure to CCHFV, as post-slaughter acidification will normally inactivate virus present (8). There have, however, been reported human cases of infection following the consumption of raw meat and liver (27) from freshly slaughtered infected animals. Pre-slaughter stress in animals can reduce the extent of post-slaughter acidification. Whether the virus can be transmitted via milk is uncertain (28), but unpasteurised milk has been suggested as a possible route of exposure (29).
While CCHFV has been detected in many tick species, only some ticks have been confirmed as competent vectors (30). Ticks of the genus Hyalomma are the principal source of human infection and the key Hyalomma vectors involved vary geographically (1). Some species of the genus Dermacentor and Rhipicephalus have also been shown to be capable of transmitting CCHFV (26), but their role in maintaining active foci is debated (31). In Spain, CCHFV has been detected in H. lusitanicum, H. marginatum, and D. marginatus (17, 32).
Infection within generations of key Hyalomma vectors is maintained by transstadial (between stages) and transovarial transmissions (in other words, by vertical transmission). The virus can be maintained for extended periods via these routes, even in the absence of susceptible vertebrate hosts (26).
Is the disease endemic in humans within the UK?
Outcome
No.
Quality of evidence
Good.
Travel associated human CCHF cases have very rarely been confirmed in the UK. Only 2 laboratory-confirmed clinical cases have been diagnosed; one imported from Afghanistan in 2012 (33) and the other from Bulgaria in 2014 (34). No onward transmission resulted from either case.
Is the disease endemic in animals within the UK?
Outcome
No.
Quality of evidence
Good.
The virus has not been found in UK mammals. Relevant tick species have been detected in the UK; there have been 2 reports of UK acquired Hyalomma ticks on hosts without a history of travel (35, 36), and occasional reports of importations via migratory birds (37) or imported animals (38). A distribution map for H. marginatum in Europe is shown below (Figure 1).
Figure 1. The known distribution of Hyalomma marginatium and Hyalomma lusitanicum in Europe and its neighbouring countries, as of October 2023. Source: European Centre for Disease Prevention and Control. Accessed: 22 January 2024
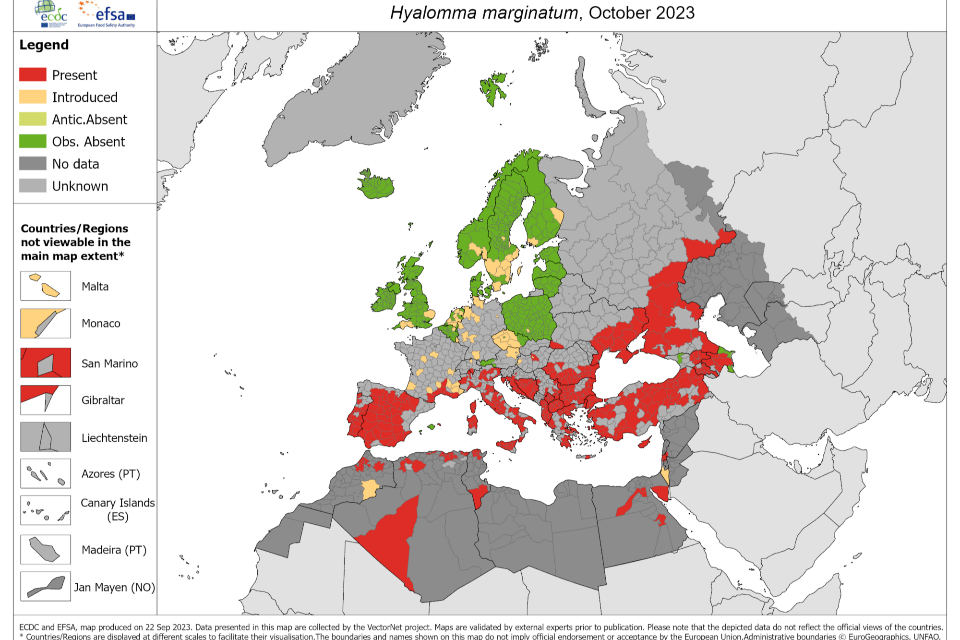
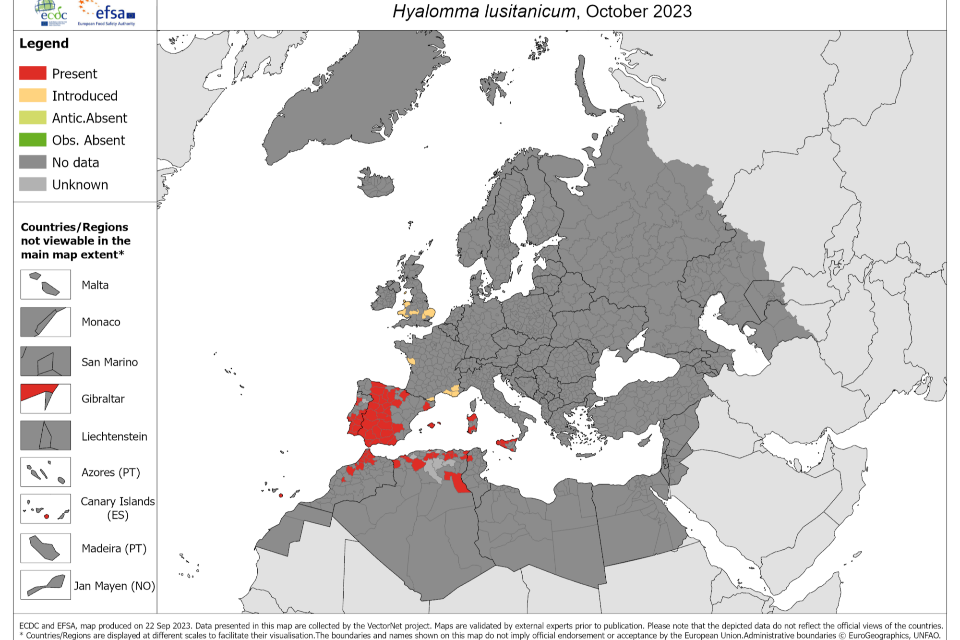
Historical tick records from the UK (39) have demonstrated that importations of H. marginatum have occurred via migratory birds, although a study in 2004 (40) collected 38 ticks from more than 10,000 birds examined, and none were Hyalomma. A subsequent UK study carried out in the spring of 2010 and 2011 examined 971 migratory birds of 29 species (30). From 53 infested birds of 9 species, 68 ticks were recovered. These were mostly Ixodes species (54/68, 79%), but 21% (14/68) were H. marginatum. The H. marginatum were found on 4 species of bird and all ticks were negative for CCHFV by PCR (30). As of January 2024, 15 Hyalomma ticks have been received by UKHSA’s passive Tick Surveillance Scheme (TSS) (Table 1).
Table 1. Hyalomma ticks received by UKHSA’s passive Tick Surveillance Scheme (TSS), as of January 2024.
| Tick species | Year | Host species | reference |
|---|---|---|---|
| H. marginatum | 2009 | Horse | (41) |
| H. lusitanicum | 2016 | Dog | (38) |
| H. lusitanicum | 2017 | Human | TSS data |
| H. truncatum | 2017 | Human | TSS data |
| H. lusitanicum | 2018 | Dog | TSS data |
| H. rufipes | 2018 | Horse | (36) |
| H. marginatum | 2018 | Human | (35) |
| H. lusitanicum | 2019 | Human | TSS data |
| H. lusitanicum | 2019 | Human | TSS data |
| H. aegypticum | 2019 | Human | TSS data |
| H. aegypticum | 2020 | Human | TSS data |
| H. lusitanicum | 2022 | Human | TSS data |
| H. marginatum | 2022 | Horse | TSS data |
| H. excavatum | 2023 | Human | TSS data |
| H. aegypticum | 2023 | Human | TSS data |
All but the H. rufipes (on a horse) and the H. marginatum (on a human) were linked to overseas travel to Hyalomma-endemic regions. The H. marginatum found on a horse in 2022 may have been acquired in the UK, but this was not confirmed. The detection of an adult male H. rufipes tick on a horse in Dorset and an adult male H. marginatum tick on a human in Norfolk, both with no history of travel, were possibly the result of importation of a nymph on a migratory bird, and subsequent moulting to an adult during the unusually warm summer of 2018 (35, 36). CCHFV was not detected in either tick (or the 2 ticks imported on dogs).
To date there is no evidence that any imported Hyalomma ticks have led to established populations in the UK.
Are there routes of introduction into animals in the UK?
Outcome
Yes.
Quality of evidence
Satisfactory.
There are routes of introduction of CCHFV into the UK via tick infested bird migration and animal movements. The latter route does not appear to have been commonly documented (25), although in the UK, Hyalomma tick species have been detected on an imported horse (41), 2 travelling dogs (38) and humans (TSS data) (Table 1).
In 2021, Fanelli and Buonavogilia assessed the likelihood of entry of CCHFV (through infected tick vectors, wildlife and livestock movements) into 9 CCHF-free countries in Southern and Western Europe (Austria, Belgium, France, Germany, Italy, Luxembourg, Netherlands, Slovenia and Switzerland). The likelihood of CCHFV entry was assessed as low to medium for most countries, with the exception of France, where the risk was considered high (42).
Livestock were, until January 2021, traded between European Union (EU) member states and the UK on an Intra Trade Animal Health Certificate (ITAHC), which has no specific requirements for CCHF freedom and no testing or tick treatment requirements. Horses were moved – until January 2021 – using either an ITAHC, or an owner health attestation, or on a commercial document for registered horses. Importation from outside the EU is strictly controlled, with few countries approved for trade in live ruminant livestock (206/2010/EU) and few approved bodies for trade in exotic ruminants. None of the approved third countries have endemic CCHF. Horses can, however, enter the UK from many third countries – including the Middle East and North Africa – and from January 2021 this includes the EU, and there are no tick treatment requirements on the Export Health Certificate. Nevertheless, since 2022, a vector borne disease of ruminants, Epizootic Haemorrhagic Disease, has been detected in Spain and France, and there is a bluetongue (BTV-3) epizootic in the Netherlands, Belgium and Germany, which means there are no imports of cattle or deer or small ruminants from these regions.
Jameson and others estimated that there could be tens of thousands of H. marginatum being imported into the UK annually via birds migrating from Africa (30). However, no established populations are known to have resulted from importation of this nature. Additionally, it has previously been considered that there is a climatic limitation with the UK being too wet or too cold during the summer potentially preventing Hyalomma development from nymph to adult. However, during periods with increased summer temperatures such as those experienced during 2018 (43), nymphal moulting may take place in the UK, as suggested by the 2018 detections of a male H. rufipes on an horse in Dorset and a male H. marginatum on a human in Norfolk; both with no history of travel. CCHFV was not detected in either tick (35, 36). However, model predictions by Gillingham and others suggest that whilst future autumn temperatures will increase in the UK, they are likely to remain below the threshold required for Hyalomma marginatum populations to become established (37).
Gale and others modelled the absolute risk of CCHFV infections of livestock through immature ticks via migratory birds as being very low (44). An assessment of the risk that ticks on northward migrating birds present to Great Britain was conducted in Spain and examined 564 birds. Overall, 65 Hyalomma tick species individuals were found on 26 birds (2.2%), none of which were positive for CCHFV (45). Tick infestation rates on birds entering the UK is estimated at <1%, and such migratory birds are considered more likely to have originated in north Africa or southern Europe. The conclusion was that migratory birds present an extremely low but not negligible risk of CCHFV being introduced into Great Britain.
Hyalomma lusitanicum has been imported into the UK on 6 occasions (2016 to 2023) on dogs and humans, each with a recent history of travel to Portugal, Spain, Menorca or Malta (38) (TSS data). These importations are considered likely to be rare events. The role of this tick species in transmission of CCHFV is unclear, though it was found to harbour CCHFV in Spain (11).
Rodents and lagomorphs are also imported into the UK, mostly as pets or for the pet trade; again, there are no requirements for tick treatment and no reports of Hyalomma tick infestations. While the host preferences for Hyalomma ticks are generally considered to be rodents, lagomorphs and avian species (46), other hosts are possible, if rare. For example, Spur-thighed tortoises (Testudo graeca) imported into the UK were found to be infested with H. aegyptium (47), a species known to be infected with CCHFV in Turkey and Syria; however, this tick species is not known to be associated with virus transmission. A large variety of reptiles are imported into the UK for the pet trade and there are no requirements for health certification or tick treatment.
Although only 2 CCHF travel-associated human cases have been reported in the UK to date, it is possible additional travel associated cases have gone undetected due to the asymptomatic or mild nature of some disease manifestations.
Are effective control measures in place to mitigate against these routes of vector introduction?
Outcome
No.
Quality of evidence
Satisfactory.
Effective control of introduction is not possible due to the role of migratory birds. There are no statutory controls for ticks on imported livestock or other hoof-stock. There are no tick controls in the Pet Travel Scheme passport or for commercial pets. Tick treatment in pets is voluntary, but it is recommended to all pet owners by the British Small Animal Veterinary Association whether their cat or dog is travelling or not (49). There are no controls for wild birds.
The presence of a viraemia due to CCHFV in an animal species would most likely be silent, thus importation of infected animals could pass any veterinary pre-movement inspection. However, there are controls on trading of livestock animals within and between EU member states and on importation from outside the EU, relating to the disease-free status of the establishment and the region, albeit not specifically related to CCHF. Importation from many CCHFV-endemic countries is not allowed, due to EU legislative requirements, based on the status of other more important livestock diseases in the country.
Do environmental conditions in the UK support the natural vectors of disease?
Outcome
Predominantly no, with current climate in the UK being considered too wet and cold for nymph survival. However, during unusually warm summers (as observed in 2018), conditions may facilitate nymph survival and moulting.
Quality of evidence
Satisfactory.
There have been no established Hyalomma tick populations detected in the UK, despite presumed frequent incursions via migratory birds. This is considered to be likely due to a climatic limitation, with the UK being too wet or too cold during the summer for Hyalomma development (26), and too cold during autumn to facilitate nymph survival (37).The recent detections of locally acquired Hyalomma ticks with no history of travel suggests that increased summer temperatures may support nymphal moulting in the UK following introduction via migratory birds. However, the lack of establishment of this tick in other parts of Europe with similar climatic conditions (see Figure 1) suggests that survival in the UK may be restricted. Model predictions by Gillingham and others suggest that whilst future autumn temperatures will increase in the UK, they are likely to remain below the threshold required for Hyalomma marginatum populations to become established (37).
Will there be human exposure?
Outcome
No, not currently
Quality of evidence
Good.
There is no evidence to suggest CCHFV is present in the UK and, to date, only a limited number of imported Hyalomma species have been detected, with no indication of establishment.
Future vector incursions and travel-associated human infections should not be unexpected, particularly as the geographical distribution of CCHFV expands (for example, into areas frequented by UK travellers including Spain and France). The possibility of human-to-human transmission following a sporadic imported human case into the UK is plausible.
Outcome of probability assessment
The probability of human infection with CCHF in the UK population is considered very low.
Step 2: Assessment of the impact on human health
The scale of harm caused by the infectious threat in terms of morbidity and mortality depends on spread, severity, availability of interventions and context. Please read in conjunction with the Impact algorithm.
Is there human-to-human spread of this pathogen?
Outcome
Yes, however the predominant route of transmission is via the bite of an infected tick
Quality of evidence
Good.
The most common route of acquisition of CCHF is tick-borne or via direct contact with blood or tissues of an infected animal during and immediately after slaughter (22). However, person-to-person transmission can occur via contact with the blood and/or body fluids of infected persons (1). Nosocomial transmission is known to occur and has been well-documented in many countries (49), including in Spain in 2017 (50) and North Macedonia in 2023 (51), but has not occurred in the UK.
Possible sexual transmission and vertical transmission have also been described (52 to 54).
Is there zoonotic or vector-borne spread of this pathogen?
Outcome
Yes.
Quality of evidence
Good.
CCHF is primarily a tick-borne infection, but disease may also be acquired via contact with the blood and/or body fluids of infected animal or human cases (1). There have also been reported human cases of infection following the consumption of raw meat and liver from freshly slaughtered infected animals (27).
For zoonoses or vector-borne disease, is the animal host or vector present in the UK?
Outcome
Yes, animal hosts are present in the UK. However, there are no established Hyalomma tick populations in the UK.
Quality of evidence
Good.
The virus is not found in UK animals and there are no established Hyalomma tick populations in the UK. However, CCHFV has a broad host range, including livestock animals, which are present in the UK.
The incidents of locally acquired adult Hyalomma ticks during 2018, on a human and a horse with no history of travel, suggest moulting of nymphs has taken place in the UK in these instances, potentially associated with the unusually warm summer of 2018 (43). Winter temperatures in the UK may not be a limiting factor for continued survival of adults, which could therefore result in questing of adult Hyalomma next spring. It is possible that future human exposure to Hyalomma ticks in the UK could occur following warm summers permissive for nymphal moulting following importation of ticks.
Is the UK human population susceptible?
Outcome
Yes.
Quality of evidence
Good.
There is no reason to suspect that the UK population is any different in susceptibility to CCHFV than populations in endemic countries. Two UK travellers to date have acquired confirmed clinical CCHF overseas, as have a number of other tourists from other countries; more than 20 travel-related cases have been documented in the literature (55, 56).
Does it cause severe disease in humans?
Outcome
Yes.
Quality of evidence
Good.
Although asymptomatic infection occurs, it is not clear what proportion of infections lead to overt clinical disease. However, when it occurs, CCHF can be a severe illness with a high mortality. In endemic countries, the case fatality rate is variable, ranging from 5% to more than 40% (1). In a series of travel-related infections, 12 out of 21 cases (57%) had a fatal outcome (55).
Would a significant number of people be affected?
Outcome
No.
Quality of evidence
Satisfactory.
The burden of disease due to CCHFV even in endemic countries appears to be comparatively low, although there is great variation in surveillance, detection and reporting.
In the UK, it is unlikely that a significant number of cases would occur from environmental exposures. There are robust and tested procedures for managing and caring for patients with viral haemorrhagic fevers (57). However, it is possible that delayed diagnoses in healthcare settings could have substantial operational impacts if staff exposed to a case between the time of admission and confirmation of infection are required to isolate as part of public health follow-up. There has been no transmission to healthcare workers involved in the care of patients with any viral haemorrhagic fever in the UK. However, nosocomial transmission has been documented in other countries (58), highlighting the importance of CCHF awareness amongst healthcare professionals to ensure rapid diagnoses and the implementation of effective infection prevention and control in healthcare settings.
Are effective interventions available?
Outcome
Yes. Whilst non-pharmaceutical interventions are available, there are no specific licensed vaccines or antivirals to treat CCHF.
Quality of evidence
Satisfactory.
Preventive measures in endemic countries focus on education around tick avoidance (59), treatment of livestock to control tick infestations, minimising contact with blood and/or body fluids of livestock animals (55) and infection prevention and control measures in healthcare settings (49).
There are no licensed vaccines or specific antivirals to treat CCHF. In recent years, a better understanding of the function of CCHFV proteins in viral replication and improved animal models have provided important insights into CCHFV pathogenesis, thus enabling preclinical testing of multiple vaccine platforms and therapeutic strategies for CCHF (60). However, this is still an area of development. The use of the antiviral ribavirin for treatment may be beneficial, provided it is commenced early in the course of illness (1) but recently the evidence for this has been increasingly challenged and more work is needed to find the best treatment regimen. Ribavirin has also been suggested as post-exposure prophylaxis following percutaneous exposure (61). Favipiravir showed good activity in treating bunyavirus infections in laboratory animals, but currently there are no data available on its efficacy in humans (62).
Treatment of a case in the UK would be supportive and include symptom relief, replacing blood components, balancing fluids and electrolytes and maintaining oxygen status and blood pressure (57, 63).
Intensive public messaging about preventive measures would take place in response to the first detection of established populations of Hyalomma ticks in the UK, the first detection of CCHFV in a tick in the UK and the first detection of a locally acquired human case.
Outcome of impact assessment
The impact of CCHF on human health in the UK is considered very low to moderate.
Annexe A. Assessment of the probability of infection in the UK population algorithm

Annexe B. Assessment of the probability of infection in the UK population algorithm: accessible text version
Outcomes are specified with (Outcome) beside the appropriate answer.
Question 1: Is this a recognised human disease?
Yes
Go to question 3. (Outcome)
No
Go to question 2.
Question 2: Is this a zoonosis or is there a zoonotic potential?
Yes
Go to question 4.
No
The probability of infection in the UK population is considered very low.
Question 3: Is this disease endemic in humans within the UK?
Yes*
Go to question 5.
No
Go to question 4. (Outcome)
*This pathway considers reverse-zoonosis of a pathogen already in circulation in the human population.
Question 4: Is this disease endemic in animals in the UK?
Yes
Go to question 8.
No
Go to question 5. (Outcome)
Question 5: Are there routes of introduction into animals in the UK?
Yes
Go to question 6. (Outcome)
No
The probability of infection in the UK population is considered very low.
Question 6: Are effective measures in place to mitigate against these?
Yes
The probability of infection in the UK population is considered very low.
No
Go to question 7. (Outcome)
Question 7: Do environmental conditions in the UK support the natural vectors of disease?
Yes
Go to question 8. (Outcome)
No
The probability of infection in the UK population is considered very low. (Outcome)
Question 8: Will there be human exposure?
Yes
Go to question 9.
No
The probability of infection in the general UK population is considered very low. (Outcome)
Question 9: Are humans highly susceptible?**
Yes
Go to question 10.
No
The probability of infection in the UK population is considered low.
**Includes susceptibility to animal-derived variants
Question 10: Is the disease highly infectious in humans?
Yes
The probability of infection in the UK population is considered high.
No
The probability of infection in the UK population is considered moderate.
Annexe C. Assessment of the impact on human health algorithm

Annexe D. Assessment of the impact on human health algorithm: accessible text version
Outcomes are specified by (Outcome) beside the appropriate answer.
Question 1: Is there human-to-human spread?
Yes
Go to question 4. (Outcome)
No
Go to question 2. (Outcome)
Question 2: Is there zoonotic or vector-borne spread?
Yes
Go to question 3. (Outcome)
No
The impact of infection in the UK population is considered very low.
Question 3: For zoonoses or vector-borne disease, is the animal host or vector present in the UK?
Yes
Go to question 4. (Outcome)
No
The impact of infection in the UK population is considered very low. (Outcome)
Question 4: Is the human population susceptible?
Yes
Go to question 5. (Outcome)
No
The impact of infection in the UK population is considered very low.
Question 5: Does it cause severe disease in humans?
Yes
Go to question 8. (Outcome)
No
Go to question 6.
Question 6: Is it highly infectious to humans?
Yes
Go to question 9.
No
Go to question 7.
Question 7: Are effective interventions available?
Yes
The impact of infection in the general UK population is considered very low.
No
The impact of infection in the general UK population is considered low.
Question 8: Would a significant* number of people be affected?
Yes
Go to question 10.
No
Go to question 9. (Outcome)
*This question has been added to differentiate between those infections causing severe disease in a handful of people and those causing severe disease in larger numbers of people. ‘Significant’ is not quantified in the algorithm but has been left open for discussion and definition within the context of the risk being assessed.
Question 9: Are effective interventions available?
Yes
The impact of infection in higher-risk groups is considered low. (Outcome)
No
The impact of infection in higher-risk groups is considered moderate. (Outcome)
Question 10: Is it highly infectious to humans?
Yes
Go to question 12.
No
Go to question 11.
Question 11: Are effective interventions available?
Yes
The impact of infection in the UK population is considered moderate.
No
The impact of infection in the UK population is considered high.
Question 12: Are effective interventions available?
Yes
The impact of infection in the UK population is considered high.
No
The impact of infection in the UK population is considered very high.
References
-
Bente DA, Forrester NL, Watts DM and others. ‘Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity’. Antiviral Research 2013: volume 100, issue 1, pages 159 to 189. DOI: 10.1016/j.antiviral.2013.07.006
-
Cross RW, Prasad AN, Borisevich V and others. ‘Crimean-Congo hemorrhagic fever virus strains Hoti and Afghanistan cause viremia and mild clinical disease in cynomolgus monkeys’. PLOS Neglected Tropical Diseases 2020: volume 14, issue 8, e0008637. DOI: 10.1371/journal.pntd.0008637
-
Messina JP, Pigott DM, Duda KA and others. ‘A global compendium of human Crimean-Congo haemorrhagic fever virus occurrence’. Nature Scientific Data 2015: volume 2, 150016. DOI: 10.1038/sdata.2015.16
-
Hawman DW and Feldmann H. ‘Crimean-Congo haemorrhagic fever virus’. Nature reviews microbiology 2023: volume 21, pages 463 to 477. DOI: 10.1038/s41579-023-00871-9
-
Garrison AR, Alkhovsky SV, Avsic-Zupanc T and others. ‘ICTV Virus Taxonomy Profile: Nairoviridae’. Journal of General Virology 2020: volume 101, issue 8, pages 798 to 799. DOI: 10.1099/jgv.0.001485
-
Lukashev AN, Klimentov AS, Smirnova SE and others. ‘Phylogeography of Crimean Congo Hemorrhagic Fever Virus’. PLOS One 2016: volume 11, issue 11, e0166744. DOI: 10.1371/journal.pone.0166744
-
Zehender G, Ebranati E, Shkjezi R and others. ‘Bayesian phylogeography of Crimean-Congo hemorrhagic fever virus in Europe’. PLOS One 2013: volume 8, issue 11, e79663. DOI: 10.1371/journal.pone.0079663
-
European Centre for Disease Prevention and Control. ‘Factsheet about Crimean-Congo haemorrhagic fever’. 2022 (viewed 18 January 2024)
-
UK Health Security Agency. ‘High consequence infectious disease: country specific risk’. 2023 (viewed 14 February 2024)
-
European Centre for Disease Prevention and Control. ‘Cases of Crimean-Congo haemorrhagic fever in the EU/EAA, 2013-present’. 2024 (viewed 18 January 2024)
-
Estrada-Pena A, Jameson L, Medlock JM and others. ‘Unravelling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: a gap analysis for the western Palearctic’. Vector Borne Zoonotic Diseases 2012: volume 12, issue 9, pages 743 to 752. DOI: 10.1089/vbz.2011.0767
-
European Centre for Disease Prevention and Control. ‘Rapid risk assessment: Crimean-Congo haemorrhagic fever in Spain - 9 September 2016’. 2016 (viewed 14 February 2024)
-
Estrada-Pena A, Palomar AM, Santibáñez P and others. ‘Crimean-Congo haemorrhagic fever virus in ticks, Southwestern Europe, 2010’. Emerging Infectious Diseases 2012: volume 18, issue 1, pages 179 to 180. DOI: 10.3201/eid1801.111040
-
Juanes HML, Carbonell C, Sendra BF and others. ‘Crimean-Congo Hemorrhagic Fever, Spain, 2013–2021’. Emerging Infectious Diseases 2023: volume 29, issue 2, pages 252 to 259. DOI: 10.3201/eid2902.220677
-
Monsalve Arteaga L, Bellido JLM, Lista MCV and others. ‘Crimean-Congo haemorrhagic fever (CCHF) virus-specific antibody detection in blood donors, Castile-Leon, Spain, summer 2017 and 2018’. Eurosurveillance 2020: volume 25, issue 10. DOI: 10.2807/1560-7917.ES.2020.25.10.1900507
-
Monsalve Arteaga L, Bellido JLM, Negredo AI and others. ‘New circulation of genotype V of Crimean-Congo haemorrhagic fever virus in humans from Spain’. PLOS Neglected Tropical Diseases 2021: volume 15, issue 2, e0009197. DOI: 10.1371/journal.pntd.0009197
-
Moraga-Fernandez A, Ruiz-Fons F, Habela MA and others. ‘Detection of new Crimean-Congo haemorrhagic fever virus genotypes in ticks feeding on deer and wild boar, Spain’. Transboundary and Emerging Diseases 2020: volume 68, issue 3, pages 993-1000. DOI: 10.1111/tbed.13756
-
Miniserio de Sanidad. ‘Evaluación rápida de riesgo: Detección de casos de Fiebre hemorrágica de Crimea-Congo en Salamanca’. 2020 (viewed 15 February 2024)
-
Santé publique France. ‘Fièvre Hémorragique de Crimée-Congo: première détection du virus sur des tiques collectées dans des élevages bovins dans le sud de la France’. 2023 (viewed 15 February 2024)
-
Messina JP and William Wint GR. ‘The spatial distribution of Crimean-Congo haemorrhagic fever and its potential vectors in Europe and beyond’. Insects 2023: volume 14, issue 9, page 771. DOI: 10.3390/insects14090771
-
Messina JP, Pigott DM, Golding N and others. ‘The global distribution of Crimean-Congo hemorrhagic fever’. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015: volume 109, issue 8, pages 503 to 513. DOI: 10.1093/trstmh/trv050
-
World Health Organization. ‘Fact sheet: Crimean-Congo haemorrhagic fever’. 2022 (viewed 18 January 2024)
-
Howard CR. Crimean-Congo haemorrhagic fever, in Viral Haemorrhagic Fevers, Perspective in Medical Virology II, A.J. Zuckerman and I.K. Mushahwar, Editors. 2005, Elsevier
-
Alavi-Naini R, Moghtaderi A and Metanat M. ‘An unusual intracerebral haemorrhage’. Canadian Journal of Infectious Diseases and Medical Microbiology 2004: volume 15, issue 3, pages 175 to 177. DOI: 10.1155/2004/769260
-
Spengler JR, Bergeron E and Rollin PE. ‘Seroepidemiological Studies of Crimean-Congo Hemorrhagic Fever Virus in Domestic and Wild Animals’. PLOS Neglected Tropical Diseases 2016: volume 10, issue 1, e0004210. DOI: 10.1371/journal.pntd.0004210
-
European Food Safety Authority. ‘Scientific Opinion on the Role of Tick Vectors in the Epidemiology of Crimean-Congo Hemorrhagic Fever and African Swine Fever in Eurasia’. 2010 (viewed 15 February 2024)
-
Sharifi Mood B, Metanat M, Hashemi-Shahri SM and others. ‘Crimean-Congo Hemorrhagic Fever Following Consumption of Uncooked Liver: Case Series Study’. Iranian Journal of Clinical Infectious Diseases 2011: volume 6, issue 3, pages 128 to 130.
-
The Centre for Food Security and Public Health. ‘Crimean-Congo Haemorrhagic Fever’. 2019 (viewed 22 January 2024)
-
United States Centers for Disease Control and Prevention. ‘Viral Hemorrhagic Fevers: CDC Yellow Book 2024’. 2023 (viewed 22 January 2024)
-
Jameson LJ, Morgan PJ, Medlock JM and others. ‘Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds’. Ticks and Tick-borne Diseases 2012: volume 3, issue 2, pages 95 to 99. DOI: 10.1016/j.ttbdis.2011.12.002
-
Gargili A, Estrada-Peña A, Spengler JR and others. ‘The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies’. Antiviral Research 2017: volume 144, pages 93 to 119. DOI: 10.1016/j.antiviral.2017.05.010
-
Negredo A, Habela MÁ, Ramírez de Arellano E and others. ‘Survey of Crimean-Congo Hemorrhagic Fever Enzootic Focus, Spain, 2011 to 2015’. Emerging Infectious Diseases 2019: volume 25, issue 6, pages 1177 to 1184. DOI: 10.3201/eid2506.180877
-
Roy KM, Ahmed S, Inkster T and others. ‘Managing the risk of viral haemorrhagic fever transmission in a non-high-level intensive care unit: experiences from a case of Crimean-Congo haemorrhagic fever in Scotland’. Journal of Hospital Infection 2016: volume 93, issue 3, pages 304 to 308. DOI: 10.1016/j.jhin.2016.02.023
-
Lumley S, Atkinson B, Dowall SD and others. ‘Non-fatal case of Crimean-Congo haemorrhagic fever imported into the United Kingdom (ex Bulgaria), June 2014’. Eurosurveillance 2014: volume 19, issue 30, 20864. DOI: 10.2807/1560-7917.ES2014.19.30.20864
-
McGinley L, Hansford KM, Cull B and others. ‘First report of human exposure to Hyalomma marginatum in England: Further evidence of a Hyalomma moulting event in north-western Europe?’. Ticks and Tick-borne Diseases 2020: volume 12, issue 1, 101541. DOI: 10.1016/j.ttbdis.2020.101541
-
Hansford KM, Carter D, Gillingham EL and others. ‘Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom?’. Ticks and Tick-borne Diseases 2019: volume 10, issue 3, pages 704 to 708. DOI: 10.1016/j.ttbdis.2019.03.003
-
Gillingham EL, Medlock JM, Macintyre H and others. ‘Modelling the current and future temperature suitability of the UK for the vector Hyalomma marginatum (Acari: Ixodidae)’. Ticks and Tick-borne Diseases 2023: volume 14, issue 2, 102112. DOI: 10.1016/j.ttbdis.2022.102112
-
Hansford KM, Medlock JM, Atkinson B and others. ‘Importation of a Hyalomma lusitanicum tick into the UK on a dog’. Vet Record 2016: volume 179, issue 16, page 415. DOI: 10.1136/vr.i5645
-
Martyn KP. ‘Provisional Atlas of the Ticks (Ixodidae) of British Isles’. Biological Records Centre. Institute of Ecology, Cumbria, UK, 1988 (viewed 16 February 2024)
-
Pietzsch ME, Mitchell R, Jameson LJ and others. ‘Preliminary evaluation of exotic tick species and exotic pathogens imported on migratory birds into the British Isles’. Veterinary Parasitology 2008: volume 155, issue 3, pages 328 to 332. DOI: 10.1016/j.vetpar.2008.05.006
-
Jameson LJ and Medlock JM. ‘Results of HPA tick surveillance in Great Britain’. Vet Record 2009: volume 165, issue 5, page 154. DOI: 10.1136/vr.165.5.154-a
-
Fanelli A and Buonavoglia D. ‘Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF-free countries in southern and Western Europe: A semi-quantitative risk assessment’. One Health. 2021: volume 7, issue 13, 100290. DOI: 10.1016/j.onehlt.2021.100290
-
Met Office. ‘UK climate anomaly maps relative to 1961 to 1990 average’. 2018 (viewed 16 February 2024)
-
Gale P, Stephenson B, Brouwer A and others. ‘Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds’. Journal of Applied Microbiology 2012: volume 112, issue 2, pages 246 to 257. DOI: 10.1111/j.1365-2672.2011.05203.x
-
England ME, Phipps P, Medlock JM and others. ‘Hyalomma ticks on northward migrating birds in southern Spain: Implications for the risk of entry of Crimean-Congo haemorrhagic fever virus to Great Britain’. Journal of Vector Ecology 2016: volume 41, issue 1, pages 128 to 134. DOI: 10.1111/jvec.12204
-
Spengler JR and Estrada-Peña A. ‘Host preferences support the prominent role of Hyalomma ticks in the ecology of Crimean-Congo hemorrhagic fever’. PLOS Neglected Tropical Diseases 2018: volume 8, issue 12, e000248. DOI: 10.1371/journal.pntd.0006248
-
Phipps LP, Johnson N, Gale P and others. ‘Potential pathway for Crimean Congo haemorrhagic fever virus to enter the UK’. Vet Record 2014: volume 175, issue 4, pages 100 to 101. DOI: 10.1136/vr.g4632
-
British Small Animal Veterinary Association. ‘BSAVA provides Q&A advice to help prevent tick bites’. 2020 (viewed 22 January 2024)
-
Leblebicioglu H, Sunbul M, Guner R and others. ‘Healthcare-associated Crimean-Congo haemorrhagic fever in Turkey, 2002 to 2014: a multicentre retrospective cross-sectional study’. Clinical Microbiology and Infection 2016: volume 22, issue 4, page 387. DOI: 10.1016/j.cmi.2015.11.024
-
Gobierno de Espana. ‘Detección de casos de Fiebre hemorrágica de Crimea-Congo en el Bierzo (León)’. 2022 (viewed 16 February 2024)
-
Jakimovski D, Grozdanovski K, Rangelov G and others. ‘Cases of Crimean-Congo haemorrhagic fever in North Macedonia, July to August 2023’. Eurosurveillance 2023: volume 28, issue 34, 2300409. DOI: 10.2807/1560-7917.ES.2023.28.34.2300409
-
Ahmeti S, Berisha L, Halili B and others. ‘Crimean-Congo Hemorrhagic Fever, Kosovo, 2013–2016’. Emerging Infectious Diseases 2019: volume 25, issue 2, page 321. DOI: 10.3201/eid2502.171999
-
Ergonul O, Celikbas A, Yildirim U and others. ‘Pregnancy and Crimean-Congo haemorrhagic fever’. Clinical Microbiology and Infection 2010: volume 16, issue 6, pages 647 to 650. DOI: 10.1111/j.1469-0691.2009.02905.x
-
Pshenichnaya NY, Sydenko IS, Klinovaya EP and others. ‘Possible sexual transmission of Crimean-Congo hemorrhagic fever’. International Journal of Infectious Diseases 2016: volume 45, pages 109 to 111. DOI: 10.1016/j.ijid.2016.02.1008
-
Leblebicioglu H, Ozaras R, Fletcher TE and others. ‘Crimean-Congo haemorrhagic fever in travellers: A systematic review’. Travel Medicine and Infectious Diseases 2016: volume 14, issue 2, pages 73 to 80. DOI: 10.1016/j.tmaid.2016.03.002
-
Papa A, Markatou F, Maltezou HC and others. ‘Crimean-Congo haemorrhagic fever in a Greek worker returning from Bulgaria, June 2018’. Eurosurveillance 2018: volume 23, issue 35, 1800432. DOI: 10.2807/1560-7917.ES.2018.23.35.1800432
-
Department of Health Advisory Committee on Dangerous Pathogens. ‘Management of Hazard Group 4 viral haemorrhagic fevers and similar human infectious diseases of high consequence: November 2015’. 2015 (viewed 16 February 2024)
-
Tsergouli K, Karampatakis T, Haidich AB and others. ‘Nosocomial infections caused by Crimean-Congo haemorrhagic fever virus’. Journal of Hospital Infection 2020: volume 105, issue 1, pages 43 to 52. DOI: 10.1016/j.jhin.2019.12.001
-
UK Health Security Agency. ‘Crimean-Congo haemorrhagic fever: origins, reservoirs, transmission and guidelines’. 2022 (viewed 22 January 2024)
-
Hawman DW and Feldmann H. ‘Crimean–Congo haemorrhagic fever virus’. Nature Reviews Microbiology 2023: volume 21, pages 463 to 477. DOI: 10.1038/s41579-023-00871-9
-
Dreshaj S, Ahmeti S, Ramadani Nand others. ‘Current situation of Crimean-Congo hemorrhagic fever in South-eastern Europe and neighbouring countries: a public health risk for the European Union?’. Travel Medicine and Infectious Diseases 2016: volume 14, issue 2, pages 81 to 91. DOI: 10.1016/j.tmaid.2016.03.012
-
Furuta Y, Takahashi K, Shiraki K and others. ‘T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections’. Antiviral Research 2009: volume 82, issue 3, pages 95 to 102. DOI: 10.1016/j.antiviral.2009.02.198
-
Rigby I, Michelen M, Dagens A and others. ‘Standard of care for viral haemorrhagic fevers (VHFs): a systematic review of clinical management guidelines for high-priority VHFs’. Lancet Infectious Diseases 2023: volume 23, issue 7, e240-e252. DOI: 10.1016/S1473-3099(22)00874-X
