Examining patent applications relating to chemical inventions
Updated 5 June 2017
Introduction
1.
These guidelines set out the practice within the Intellectual Property Office (the “Office”) as it relates to patent applications for inventions in the chemical area. The relevant legislation is the Patents Act 1977, as amended by subsequent legislation, and the Patents Rules 2007. The interpretation of this legislation has been informed by case law in the UK courts. It has also reflected the fact that judicial notice must be taken of international conventions (such as the European Patent Convention (EPC)) and of decisions and opinions made under these conventions by the appropriate bodies. Accordingly, decisions taken by the UK courts relating to the 1977 Patents Act are binding on our practice, whilst European Patent Office (EPO) Board of Appeal decisions are strongly persuasive. UK court decisions under previous legislation may also be persuasive, depending on the extent to which that aspect of patent law had been changed by the 1977 Act
Any comments or questions arising from these guidelines should be addressed to
Simon Grand
Room 3Y33
The Intellectual Property Office
Concept House
Cardiff Road
Newport
South Wales
NP10 8QQ (Telephone 01633 814964)
Gareth Prothero
Room 3B15
The Intellectual Property Office
Concept House
Cardiff Road
Newport
South Wales,
NP10 8QQ (Telephone 01633 813715)
Natalie Cole
Room 3Y33
The Intellectual Property Office
Concept House
Cardiff Road
Newport,
South Wales,
NP10 8QQ (Telephone 01633 813957)
Basic considerations
2.
The bulk of patent applications relating to chemical subject matter will be decided on the basic issues of novelty, inventive step and industrial application, as well as on the requirements that the description should be sufficient and should support the claims. The Manual of Patent Practice (the “Manual”) is the examiner’s main source of information regarding current practice in the Office under the Patents Act 1977, and these guidelines are merely intended to supplement the guidance given in the Manual. Therefore, in situations where the Manual has been updated more recently than these guidelines, the practice set out in the Manual should be followed. Chemical inventions are considered in the same light as other technical inventions; however the courts have given a great deal of consideration to the particular issues arising in chemical cases, especially those in the medical and pharmaceutical fields. Therefore these guidelines seek to aid the examiner in two ways: by highlighting relevant case law; and, where appropriate, by giving guidance on its application. The decisions highlighted are primarily those of the UK courts, but relevant EPO Board of Appeal decisions are also referred to. As noted in the previous paragraph these Board decisions are persuasive, but not binding, and in certain situations UK decisions do not allow Board decisions/EPO practice to be followed. For example, the UK courts have not endorsed the Enlarged Board of Appeal decisions in G2/88 MOBIL OIL/Friction Reducing Additive and G6/88 BAYER with regard to new technical effects of known chemical substances Suitability for use and Mobil Oil section at paragraph 32.
Novelty
3.
Section 2: Novelty of the Manual of Patent Practice sets out the practice in the UK concerning the novelty requirement under the Patents Act 1977. However, the consideration of novelty with regard to chemical inventions is worthy of particular scrutiny. For example, it is important not to confuse the objection that a chemical compound found in nature lacks novelty with the objection that the compound is non-patentable because it is merely a discovery. In essence, a natural substance which has been isolated for the first time and which had no previously recognised existence does not lack novelty simply because it has always been present in nature. [footnote 1]
“It is established patent practice to recognise the novelty for a natural substance which has been isolated for the first time and which had no previously recognised existence.
V 0008/94 Howard Florey Institute’s Application / Relaxin OJEPO 1995, 388 (at page 394)
Enabling disclosure
4.
An invention defined in a claim lacks novelty if the specified combination of features has already been anticipated and thus forms part of the state of the art. In SmithKline Beecham plc’s (Paroxetine Methanesulfonate) Patent (PDF, 130 KB) [footnote 2], the House of Lords held there were two requirements for anticipation: prior disclosure see the Manual of Patent Practice 2.03 to 2.09 and enablement Manual of Patent Practice 2.10-2.10.2. These are distinct concepts, each of which has to be satisfied and each of which has its own rules. A novelty destroying disclosure must be “enabling” if what it discloses is to be regarded as being “made available to the public”. [footnote 3]
” ……. I do not see how an invention can be said to have been made available to the public merely by a published statement of its existence, unless the method of working is so self-evident as to require no explanation.”
Asahi Kasei Kogyo KK’s Application [1991] RPC 485 (at page 539) (House of Lords)(PDF, 4.7 MB)
5.
A disclosure only destroys the novelty of a later invention if the information it contains, when understood by a person skilled in the art, is sufficient to allow reproduction of the later invention. [footnote 4] Thus a chemical compound whose name or formula is stated in a prior document is not to be considered as known unless the information provided enables that compound to be prepared, or in the case of a naturally occurring compound to be isolated (see EPO Guidelines Part G, Chapter VI, 4).
Whilst it may theoretically not be absolutely impossible to proceed on the basis of the citation, a novelty destroying document must according to standard practice, be enabling without undue burden to a person skilled in the art. In such circumstances, inventions might require an actual demonstration of reduction to practice and corresponding detailed instructions to the public in a document, to become available for the purposes of Article 54 EPC as part of the state of the art.
T 0081/87 Collaborative / Preprorennin OJEPO 1990, 250 (at page 258)
6.
It is for this reason that in general, although not exclusively, disclosures in a previous patent document impugning the novelty of a chemical invention will most readily be found in the experimental section (i.e. the examples). Similarly novelty destroying disclosures found in non-patent literature will generally be found in the results or experimental sections and the figures relating thereto.
7.
However, an earlier enabling disclosure could, in some circumstances, destroy the novelty of a later invention even if this earlier disclosure has not actually been “reduced to practice” (this term means actual embodiment of the invention and in a chemical context would generally relate to actual synthesis or testing). [footnote 5] Actual prior identification of a process or product claimed is not in itself necessary to find a lack of novelty. All that is required are instructions which, if followed, would inevitably result in the use of the claimed process or product. In SmithKline Beecham plc’s (Paroxetine Methanesulfonate) Patent,[footnote 2] the House of Lords considered that a person skilled in the art must be able to perform the invention, even if it was not precisely described in the earlier disclosure. In this case, the earlier disclosure used a solvent that was unsuitable for the crystallisation of paroxetine methanesulfonate, but a person skilled in the art would know to change the solvent in order to generate the required crystals.
If an inventor through clever foresight or lucky guess work describes something which works and how to do it, his disclosure is enabling. It is nihil ad rem that he never carried out the experiments themselves or faked the results. The more complex the area of technology, the less likely it is that the inventor will be able to predict the results of experiments he never carried out or that he will strike lucky, but what is important is what the document teaches, not how the contents got there.
Evans Medical Ltd’s Patent [1998] RPC 517 (at page 550) (Patents Court)
8.
In accordance with Toyama Chemical Co. Ltd’s Application, the Office practice in relation to a document that outlines the steps to obtain a desired end product is to assume that the disclosure is an enabling disclosure of that end product. [footnote 6] An applicant against whose application such a document is cited can challenge this assumption by argument and/or evidence. If they do, the Office will decide, on the balance of probabilities, whether the disclosure is enabling or not.
Enabling disclosure: crystalline/polymorphic forms
9.
In SmithKline Beecham plc’s (Paroxetine Methanesulfonate) Patent (PDF,130 KB), [footnote 2] it was accepted that the paroxetine methanesulfonate was monomorphic. Thus any method of producing crystals of paroxetine methanesulfonate should produce the same crystals. Where polymorphism exists this would not be the case. Therefore in a situation where a polymorphic form of a known compound is claimed, and this form of the compound has never previously been recognised or prepared, then a prior disclosure of the compound cannot generally be novelty destroying as it lacks an enabling disclosure of the particular polymorphic form. However, if the prior art teaches a method that would inevitably result in preparation of a supposedly previously unknown polymorph then there would be enablement.
10.
In practice the crystallisation methods used in the application and prior art should be compared as should the crystallographic (XRD) and IR data provided. If a prior art method according with that in the application is found or, if appropriate, a method falling within the scope of that claimed for the preparation of the particular form is found this should be cited as a prima facie novelty citation. Similarly matching IR and particularly XRD data may well suggest that the prior art relates to the same polymorph as the application being searched.
11.
The EPO boards of appeal have considered polymorphic forms on a number of occasions. Whether claims to such forms have been allowed has depended on the facts of the individual cases. Relevant cases include T 1555/12 (PDF,438 KB) [footnote 7] and T 2397/11 (PDF,608 KB) [footnote 8]. See also the section on the inventiveness of crystalline forms at paragraph 56.
Enabling disclosure: enantiomers
12.
Where a product has only been available as a racemate and not as a single enantiomer, the single enantiomer is deemed not to have been made available to the public (due to lack of an enabling disclosure) and thus does not form part of the state of the art as defined in section 2(2). The practice under UK law was established in Generics (UK) Limited and others v H Lundbeck A/S, [footnote 9] where it was held that the line followed in [SmithKline Beecham Plc’s (Paroxetine Methanesulfonate) Patent] (http://www.bailii.org/uk/cases/UKHL/2009/12.html) [footnote 2] in relation to enabling disclosures associated with crystalline forms is to be followed. This does not preclude enantiomers in most instances being rendered non-inventive by prior disclosure of the racemate (exceptions to this include the situation encountered in Generics v Lundbeck [footnote 9] where the enantiomers could not be prepared in a straightforward manner by known resolution/separation techniques). See paragraph 55 below for discussion of the inventiveness of enantiomers.
Enabling disclosure: chemical purity
13.
In general a prior art document disclosing a low molecular weight chemical compound and its manufacture is regarded as making the compound available to the public in all grades of purity (see for example Technical Board of Appeal decision T 990/96 (PDF, 36.4 KB)[footnote 10]. Therefore if a party alleges that this general rule would not be applicable in a particular case, then the burden of proving the existence of such an exceptional situation (e.g. a situation where all prior attempts to achieve a particular degree of purity by conventional purification processes have failed) lies with that party.
14.
However, the same does not necessarily hold for high molecular weight compounds e.g. proteins, polymers etc. In Synthon B.V. v Teva Pharmaceutical Industries Limited, [footnote 11] which concerned the validity of patents covering glatiramer acetate (a polymer formed from 4 different amino acids), Birss J. rejected the notion that T 990/96 (PDF, 36.4 KB) created a general legal principle that purity is not patentable both by distinguishing the case on the basis of the size of the biological molecule and also because T 990/96 (PDF, 36.4 KB) specifically accepts the possibility of exceptions.
15.
In T 803/01 (PDF, 42.3 KB) [footnote 12] the purity of components of a composition was considered (claims to a polylactide defined in terms of maximum amounts of metal ions and colouration were amended to relate to a composition comprising a polylactide with these properties and a particular active ingredient). The Board took the view that following T 990/96 (PDF, 36.4 KB) and in relation to low molecular weight compounds, “each and every purification method is presumed, provided it is ‘conventional’ but regardless of the extent of purification desired to be achieved, to be automatically available to the public, and this in a fully enabling way, so as to amount to an effective novelty-destroying disclosure”. The Board decided that on the balance of probabilities the ‘conventional’ methods of purification would not be capable of delivering the required degree of purity and thus the invention was novel. Nonetheless this establishes that the reasoning in T 990/96 (PDF, 36.4 KB) may be applied to individual components of a composition.
Prior disclosure: individualised description - small number of alternatives
16.
A generic disclosure does not impugn the novelty of a more specific claim. In some cases however the disclosure of a comparatively small and restricted group of possible alternatives may be regarded as a disclosure of each and every member of that group; for example, “fluid” may be taken to disclose both liquid and gas if the context warrants it. In Norton Healthcare Ltd v Beecham Group Plc [footnote 13] Jacob J held that a prior suggestion of a combination of sodium or potassium clavulanate with amoxycillin or ampicillin trihydrate (four possible combinations) was a disclosure of each of the combinations. A special case is the disclosure of natural extracts. In general, generic disclosure of a natural material would not be considered to impugn the novelty of a specific extracted material. However, care should be taken when the extracted material forms the bulk of the natural material and/or where there are only a very low number of significant components.
17.
A variation on this theme is that which was treated as a prior (and enabling) disclosure of atorvastatin calcium in Ranbaxy v Warner-Lambert (PDF, 36.4 KB) [footnote 14]. The prior art included a scheme disclosing preparation of a racemic carboxylic acid, there was a statement that such compounds react to form salts with pharmaceutically acceptable metal and amine cations and a list of 7 metal ions from which salts were contemplated. In addition there was a further statement that the 4R,6R-isomer was preferred. On the basis of evidence that resolution of these compounds was common general knowledge, [footnote 15] it was decided that “an expressly specified salt of the preferred isomer of one of three materials explicitly specified” represented a clear case of anticipation. In view of the statement by the Court of Appeal in General Tire & Rubber Company v Firestone Tyre & Rubber Company Limited [footnote 16] that what is required for anticipation are “clear and unmistakeable directions to do what the patentee claims to have invented”, this disclosure would appear to be approaching the extreme (in a chemical context) of what can reasonably be considered as, using the wording of Glass v Freyssinet Ltd, [footnote 17] a “clear and unambiguous teaching, taking the prior art as a whole” (see also the ‘Compositions and the EPO two-list principle’ section below).
Prior disclosure: individualised description and Markush structures
18.
Where there are more than a few alternatives such as in a Markush claim, [footnote 18] then “logic dictates rejection of the argument that a disclosure of a large class is a disclosure of each and every member of it” as Jacob LJ stated in Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly & Co Ltd[footnote 19]. In this case Jacob endorsed the EPO case law in which an “individualised description” is required for anticipation of a later claimed compound or class of compounds. [footnote 20]
6.1 Here the Board is guided by the conclusions it reached in its “Spiro compounds” decision T 181/82 (OJ EPO 1984, 401) concerning the novelty of chemical entities within a group of substances of known formula. With regard to products of the reaction of specific spiro compounds with a (C1-C4)-alkyl bromide defined as a group, the Board drew a sharp distinction between the purely intellectual content of an item of information and the material disclosed in the sense of a specific teaching with regard to technical action. Only a technical teaching of this kind can be prejudicial to novelty. If any such teaching is to apply in the case of a chemical substance, an individualised description is needed.Evans Medical Ltd’s Patent [1998] RPC 517 (at page 550) (Patents Court)
T 0296/87 HOECHST/ Enantiomers OJEPO 1990, 195 (at page 206)
19.
The practice of the EPO in this area was summarised in T 12/90. The board considered the novelty of a vast family of chemical compounds represented by a Markush structure, where the prior art also disclosed a vast family defined by a generic structural formula with considerable overlap. The board distinguished between two situations: a) If the subject matter of the invention was a particular compound, whereas the prior art disclosed a family of compounds defined by a generic structural formula including this compound but not describing it explicitly, the invention has to be considered novel (c.f. Jacob LJ’s comments in Dr Reddy’s v Eli Lilly referred to above). b) If, with the same prior art, the subject matter of the invention was a second family of compounds partially covering the first, the invention was not new.
This is further discussed in the EPO Case Law of the Boards of Appeal Section I.C 6.2.2 where the Board is stated as saying “the concept of individualisation naturally only applies to the structural definition of a single compound, not a collection of compounds.”
20.
However, it remains Office practice to cite a disclosure of a Markush structure encompassing a later claimed compound or compounds as a prima facie obviousness objection (see paragraph 90).
Prior disclosure: salts in solid and aqueous form
21.
The Technical Board of Appeal in T 352/93 [footnote 21] decided that a claim for an ionic compound (salt) that was defined only by the structural formulae of the cation and anion of the compound, was not novel over prior art disclosing an aqueous solution that contained a base corresponding to the cation and an acid corresponding to the anion.
Prior disclosure: metabolites
22.
The chemical composition of a product need not necessarily be known for it to form part of the start of the art. In Merrell Dow Pharmaceuticals Inc v H N Norton & Co Ltd [footnote 22] Lord Hoffmann held that section 2(2) does not confine the state of the art about products to knowledge of their chemical composition. The invention is part of the state of the art if the information which has been disclosed enables the public to know the product under a description sufficient to work the invention. Thus, in Merrell Dow, which centred on a claim to an acid metabolite formed in the liver after administration of terfenadine (itself the subject of an earlier patent), the acid metabolite was held to be anticipated not by prior use (see the Manual 2.29), but because it was the inevitable result of carrying out the directions in the earlier terfenadine patent.
Prior disclosure – starting material and reaction conditions
23.
Closely related to the above, T 12/81 [footnote 23] concerned whether a prior art disclosure of a list of 20 chlorophenoxy ketone starting compounds and five different well-described methods for their hydrogenation constituted a disclosure of one particular product of these combinations. The Board decided that where a cited document describes the starting ketone, it automatically co-describes the secondary alcohols in a manner analogous to a product-by-process claim. In other words, by virtue of the teaching in the cited document that chlorophenoxy ketone is to be reduced optionally by any of the five process variants indicated, the product of the process according to each of these alternative reduction methods is to be regarded as having been made available to the public by written description.
Product-by-process
24.
In Kirin-Amgen Inc. v Hoechst Marion Roussel Ltd the House of Lords [footnote 24] overturned the view of the Court of Appeal [footnote 25] that a claim to any product can be characterised by a method of producing the product, and that the product of a claimed method will be novel if that method itself is novel. As a consequence of this ruling the Office has changed its practice and now takes a similar approach to that of the EPO. [footnote 26] As a result product-by-process claims where the product is known are now rejected on the basis that the product is not novel. Therefore a claim to a product obtained or produced by a new process is anticipated by any prior disclosure of that particular product per se, regardless of its method of production.
I think it is important that the United Kingdom should apply the same law as the EPO and the other Member States when deciding what counts as new for the purposes of the EPC… It is true that this means a change in practice which has existed for many years. But the difference is unlikely to be of great practical importance because a patentee can rely instead on the process claim and article 64(2). It would be most unfortunate if we were to uphold the validity of a patent which would on identical facts have been revoked in opposition proceedings in the EPO.
Kirin-Amgen Inc. and others v Hoechst Marion Roussel Ltd and others [2005] RPC 9 (at page 200)
25.
Section 60(1)(c) of the Act, which corresponds to Article 64(2) of the EPC, states that the protection provided by a claim to a process extends to any product obtained directly by means of that process. Therefore, the patentee still has some protection for the products of his novel process under this section of the Act.
26.
The Office does allow product-by-process claims in certain circumstances, in line with practice at the EPO. A claim to a product defined by its method of production is an acceptable form of claim only when there is no physical, chemical or biological means for distinguishing that product from the prior art. Such a claim is considered to lack clarity if there is a satisfactory alternative chemical, physical or biological way of defining that product. In claims for products defined in terms of a process of manufacture the products as such must still fulfil the normal requirements for patentability; a “product-by-process” claim is not rendered novel merely by the fact that the product is produced by means of a new process. [footnote 27]
A product-by process claim is interpreted according to the jurisprudence of the Boards of Appeal as a claim directed to the product per se, since the reference to a process serves only the purpose of defining the subject matter for which protection is sought, which is a product. Whether or not the term ‘directly obtained’ or any other term , such as ‘obtained’ or ‘obtainable’ is used in a product-by-process claim, the category of that claim does not change as it is directed to a physical entity and the subject matter of that claims, for which protection is sought, remains the product per se.… Therefore, irrespective of how a product-by-process claim is worded, it is still directed to the product per se and confers absolute protection upon the product, precisely as any other claim to a product per se. That product claim, hence, confers protection upon the product regardless of the process by which it is prepared.
T 0020/94 Amorphous TPM/ ENICHEM (not reported)(PDF,1,439 KB)
27.
As product-by-process claims are considered to relate to the product per se, a claim to a product ‘obtainable’ by a process is acceptable, provided the product is new and inventive and cannot be otherwise defined. Whilst the term ‘obtainable’ does not limit the claim to a product when made by a particular process, this is not necessary because the claim is treated as a per se claim. This is consistent with Part F, Chapter IV, 4.12 of the EPO Examination Guidelines.
Selection inventions
28.
When a selection is made from a larger prior art group then the novelty of that selection is dependent on the normal considerations of novelty and the mere presence of an unexpected technical effect, despite being important in consideration of any inventive step (see below) cannot afford novelty to an otherwise anticipated selection.In Ranbaxy v Warner Lambert [footnote 14] Pumfrey J reviewed the authorities on selection inventions and quoted the EPO Technical Board of Appeal [footnote 28].
To prevent misunderstanding, it should be expressly emphasised that when examining so-called selection inventions as to novelty the Board adheres to the principle that the sub-range singled out of a larger range is new not by virtue of a newly discovered effect occurring within it, but must be new per se (cf. T12/81 BAYER/Diastereoisomers OJ EPO 8/1982 296 303). An effect of this kind is not therefore a prerequisite for novelty; in view of the technical disparity [sc. between the new class and the old] however, it permits the inference that what is involved is not an arbitrarily chosen specimen from the prior art, that is, not a mere embodiment of the prior description, but another invention (purposive selection).
T198/84 HOECHST/Thiochloroformates OJEPO 1985, 209 (at page 214).
29.
EPO Guidelines Part G-VI 8ii sets out criteria for establishing whether a sub-range is “new per se” and may be informative when assessing a range or sub-range. Care should however be taken as the third of the three criteria (i.e. the need for a technical advance and a non-arbitrary selection) establishing a novel sub-range is increasingly being dealt with under inventive step by the Boards of Appeal. In the UK, Jacob J held that a range is normally to be understood as disclosing all points falling within it, [footnote 29] however, he explicitly left open the door for selection inventions:
A prior disclosure of a range should normally be regarded as disclosing each and every part of the range. However if there is something special about a later claimed part of the range, there may be room for invention - an invention along the lines of a selection invention. But there must be something special about the later range.”
Union Carbide Corp v BP Chemicals [1998] RPC 1 (at page 15)
30.
However, Jacob LJ has since noted, in the context of Markush structures as referred to above, that “logic dictates rejection of the argument that a disclosure of a large class is a disclosure of each and every member of it”.[footnote 19] Thus the position of the UK courts with regard to ranges at least is not entirely clear.
Suitability for use
31.
A claim to a material or composition for a particular purpose is regarded as a claim to the material or composition per se. [footnote 30] Therefore if a product is known in a form suitable for a stated use then it would render a claim to the product for that use not novel even though the product has never been described for that use. By contrast, a known product which is per se the same as the material or composition defined in the claim, but which is in a form which would render it unsuitable for the stated use, would not deprive the claim of novelty. This is particular relevant for chemical compounds where the suitability of a particular compound for a particular use will be an intrinsic property. In I. G. Farbenindustrie A.G.’s Patents, [footnote 31] it was stated that “no man can have a patent merely for ascertaining the properties of a known substance”. There is however an exception to this general principle where the claim is to a known substance or composition for use in a surgical, therapeutic or diagnostic method (see section 4A of the Patents Act 1977 and the Examination Guidelines for Patent Applications relating to Medical Inventions).
Use claims and Mobil Oil
32.
Office practice is that a claim to, for example, the use of a known substance as an additive to perform a particular function, is not novel if this function was inherent (though unrecognised) in the prior art use of that substance. In contrast, the Enlarged Board of Appeal of the EPO has held in Decisions G 2/88 (PDF,1,434 KB) [footnote 32] and G 6/88 [footnote 33] that a claim to the use of a known compound for a particular purpose, which is based on a technical effect which is described in the patent, should be interpreted as including that technical effect as a functional technical feature. Accordingly the claim is not open to an objection of lack of novelty provided that the technical feature has not previously been made available to the public. In G 2/88 MOBIL OIL/Friction Reducing Additive (PDF, 1,434 KB), the new technical effect was the discovery that the claimed compound, previously used in lubricant compositions to inhibit rust, had friction reducing properties. A claim to the use of that compound in a composition for reducing friction was held to be novel even though such friction reduction had inherently occurred in its previous use. Similarly, in G 6/88 Bayer, use of certain compounds as a fungicide was held to be novel even though the method of use was identical to a known use of the compound as a plant growth regulator. However, these EPO decisions are not the current practice of the Office and should not be followed.
33.
The Office’s existing practice is supported by the recent decision of the Patents Court in Tate & Lyle Technology v Roquette Frères [footnote 34]. Although the case does not refer to G 2/88 MOBIL OIL/Friction Reducing Additive (PDF, 1,434 KB), it appears to take a different approach from the Enlarged Board to use claims based on an unrecognised technical effect. The only claim in question here was to “the use of maltotriitol to modify or control the form of maltitol crystals”. This was based on the previously unsuspected effect of an impurity (maltotriitol) on the crystallisation of the sweetener maltitol. This was held to lack novelty over a number of prior art documents which disclosed crystallisation of maltitol in the presence of maltotriitol at levels at which it would control crystal formation:
The industry has been using maltotriitol to control or determine crystal habit without knowing it. What is left of the patent as granted is no more than a discovery as such.
Tate & Lyle Technology v Roquette Frères [2010] FSR 1 (at paragraph 72)
34.
Nevertheless, a claim to an apparatus or material “when used in” a particular process is construed as a claim confined to the use of the apparatus or material in such a process, and its novelty is therefore destroyed only by a disclosure referring to such use. (If the apparatus or material per se is known to be old, this fact should be acknowledged in the specification, in order to ensure that the nature of the invention is presented in its proper perspective).
35.
It should also be noted that a claim such as “the use of substance X as an insecticide” is regarded as equivalent to a “process” claim of the form “a process of killing insects using substance X” and is not interpreted as directed to the substance X recognisable (e.g. by further additives) as intended for use as an insecticide (see also the Manual of Patent Practice 2.16).
Implicit disclosures
36.
It is normally required that, for an invention to lack novelty, the features of the claim under consideration are explicitly disclosed, for example in an earlier publication. However, the teaching implicit in a document can be taken into account, as guided by paragraph 2.07 of the Manual of Patent Practice. This may be particularly relevant in chemical process cases or cases relating to intrinsic properties of compounds or compositions etc.
Claims defined in terms of parameters
37.
A claim which defines an invention by reference to parameters, for example of a process or a product, is anticipated by a disclosure which when put into practice would necessarily fall within the scope of the claim, even if the disclosure does not refer to these particular parameters (see the Manual of Patent Practice 2.04 and 2.04.1).
38.
If an invention is defined by reference to parameters (of a material or composition) which are not usually specified in the prior art, and also by reference to a method of producing the material or composition, a search for the method per se may suffice. If the claim does not specify a method, reference data may be utilised to identify materials likely to possess the specified parameters (e.g. solvents with appropriate boiling points for a particular reaction or polymers likely to display sufficient solubility in water), with a search then being made for such materials, and/or a search may be made for disclosures of prior art materials akin to those exemplified in the application and which thus might be expected to possess the same properties. Prior disclosures of such likely anticipations should be cited. The onus of distinguishing the invention in this situation falls upon the applicant. The same procedure may be used if difficulties arise with the use of parameters in a method claim.
Compositions and the EPO two-list principle
39.
Determining whether the disclosure within one document represents a novelty destroying disclosure can be problematic (for discussion of inventive step in relation to compositions see paragraph 89 below). It is straightforward if, for instance, the whole formulation is disclosed in an example or as a list of ingredients that the skilled worker could readily combine themselves without directions how to do so. However if the disclosure of interest is distributed in various places within a document then what would the skilled worker make of it? The EPO Boards of Appeal have on occasion used T 12/81 (referred to at paragraph 23 above) as the basis of an argument that a disclosure cannot be novelty destroying if the prior art disclosure requires a selection from two relatively long lists (see for example T 401/94 relating to a polymer blend) [footnote 35] . However in Glass v Freyssinet Ltd [footnote 17] Hacon J stated “I am not sure about the two-list principle. If, for instance, a single prior art document discloses two lists of constituents and states clearly and wholly unambiguously that any one from the first list may be combined with any one from the second list, then to my mind this is exactly the same disclosure as setting out all possible combinations seriatim in a single list” and went on to state that he was bound by Ranbaxy v Warner-Lambert which “seems to have been a two-list case”. As a consequence the principle should not be relied upon as applicable to UK law. However, as with the small number of alternatives discussed at paragraphs 16-17 above, Hacon J’s words in Glass v Freyssinet Ltd are worthy of consideration:
I think that an important criterion in determining whether a combination from within a single item of prior art deprives a subsequent claim to that combination of novelty is whether there is clear and unambiguous teaching, taking the prior art as a whole, that the relevant combination can be made. Absent such a clear and unambiguous indication, the prior art will not disclose subject-matter which, if (comprehensively) performed, would necessarily result in an infringement of the patent in suit (see Smithkline Beecham’s Patent at [22]).
Hacon J in Glass & Others v Freyssinet Ltd [2015] EWHC 2972 (IPEC) (at paragraph 49)
Plausibility
40.
Neither the UK Patents Act 1977 nor the EPC make reference to plausibility. However over recent years the courts have applied it in relation to a number of areas of patent law including: sufficiency, obviousness, selection inventions, priority and industrial applicability.
41.
Carr J summed up the appropriate place of plausibility in deciding issues of patent validity in Actavis v Eli Lilly [footnote 36].
[173] …Susceptibility of industrial application, sufficiency and inventive step are all part of the requirements of the relevant treaties and legislation. Necessarily, it is the task of the courts and the Boards of Appeal of the EPO to interpret these requirements. In so doing, plausibility has been referred to at the highest level as one factor that should be taken into account. Nonetheless it is relevant to bear in mind that plausibility does not form a separate ground of objection to the validity of a patent. Therefore it falls to be considered within the context of the statutory objections to validity, and having regard to their respective purposes.
42.
Plausibility as a concept has arisen from the “problem- solution approach” adopted by the EPO, and is derived from the fundamental principle that the scope of patent monopoly must be justified by the patentee’s technical contribution to the art, that is “…a technical problem may be regarded as being solved only if it is credible that substantially all claimed embodiments exhibit the technical effect upon which the invention is based…” EPO Guidelines for Examination Part G Section VII at 5.2, Formulation of the technical problem.
43.
The key Board of Appeal decisions which first provided clarity regarding the principle that, in order to be patentable claims must make a technical contribution which is at least plausible, were T 939/92 AGREVO [footnote 37] and later T 1329/04 Johns Hopkins (PDF,82.7 KB) [footnote 38] . “The definition of an invention as being a contribution to the art, i.e. as solving a technical problem and not merely putting forward one, requires that it is at least made plausible by the disclosure in the application that its teaching solves indeed the problem it purports to solve” (Johns Hopkins T1329/04).
44.
In Conor v Angiotech, Hoffmann LJ considered AGREVO and Johns Hopkins and, whilst counselling against conflating sufficiency and obviousness, held that a specification must “pass the threshold test of disclosing enough to make the invention plausible” such that if the technical effect is plausible “the question of obviousness should not be subject to a different test according to the amount of evidence which the patentee presents to justify a conclusion that his patent will work”.
45.
This oft-repeated phrase that plausibility is a ‘threshold test’ is important and is applicable to all of the areas where plausibility has been considered by the courts. However, whilst in EPO practice this addition of a first “threshold” step which must be passed before assessment of the statutory requirements can be dealt with up front (e.g. when adopting the problem-solution approach for obviousness the extent to which an invention is plausible changes the problem to be solved), this is not the case in IPO practice in relation to inventive step (including for selection inventions) where the Windsurfing-Pozzoli test requires that the inventive concept be that determined from the claim not the specification as a whole. Thus, despite some recent court decisions, it is usual Office practice to deal with the plausibility of being able to work an invention (e.g. in terms of ability to prepare a polymer or evidence of a technical effect relied upon) across the scope of the claims as a sufficiency objection rather than as an inventive step objection (although the plausibility of a prima facie selection invention may need to be considered after application of the Windsurfing-Pozzoli test). By contrast, industrial applicability, sufficiency (in its own right) and priority do not present a problem and plausibility can be considered at the outset.
46.
In this developing area of case law, the threshold for plausibility is not as yet clearly defined. However, the “threshold is low” [footnote 39]; requires that something is “more than incredible” [footnote 39] ; must provide “some real reason for supposing that the statement is true … the standard is not any higher than that” [footnote 39]; must be “’credible’ as opposed to speculative” [footnote 40] ; and “the standard for assessment of plausibility is not the same as assessment of obviousness”. [footnote 41]
47.
Plausibility is discussed further in the context of the relevant provision under the Patents Act 1977:
- plausibility and priority (see Examination Guidelines for Patent Applications Relating to Medical Inventions paragraph 210)
- plausibility and industrial applicability (see Examination Guidelines for Patent Applications Relating to Biotechnological Inventions paragraph 60)
- interplay/interaction of plausibility and inventive step (see paragraphs 71-85)
- plausibility and inventive step (selection inventions) (see paragraph 77-85)
- plausibility and inventive step (Agrevo obviousness) (see paragraphs 75-76)
- plausibility and inventive step/sufficiency squeeze (see paragraphs 72-74)
- plausibility and sufficiency (see paragraphs 113-4)
Inventive step
48.
Section 3 of the Manual sets out the practice in the UK concerning the requirement for an inventive step under the Patents Act 1977. When determining inventive step the four steps of “Windsurfing”, [footnote 42] as reformulated in Pozzoli SPA v BDMO SA [footnote 43] are used. The four step approach of Windsurfing/Pozzoli is intended to address the concept of inventive step without the benefit of hindsight, by ensuring that the examiner assesses the invention through the eyes of the person skilled in the art, with the benefit of their common general knowledge. The inventive concept of the claim in question is then construed, and the differences between the state of the art and the inventive concept of the claim are identified. This then enables the examiner to approach the final step and ask ‘is it obvious?’. Section 3 of the Manual discusses these steps in detail, and therefore each step of this test will not be discussed at length here. Instead these Guidelines will review the requirement for an inventive step in the light of judgments of the UK courts and decisions of the EPO Boards of Appeal as they relate to chemical inventions in particular, and where appropriate by their relevance to a specific step of the Windsurfing/Pozzoli test, and should only be considered in concert with the guidance provided in the Manual.
Skilled addressee/common general knowledge
49.
The skilled person should be taken to be a worker who is aware of everything in the state of the art and who has the skill to make routine developments but not to exercise inventive ingenuity. Where the relevant skilled person is an individual, the person may be described, in terms used by Laddie J. in Pfizer’s Patent, [footnote 44] as follows:
The question of obviousness has to be assessed through the eyes of the skilled but non-inventive man in the art. This is not a real person. He is a legal creation. He is supposed to offer an objective test of whether a particular development can be protected by a patent. He is deemed to have looked at and read publicly available documents and to know of public uses in the prior art. He understands all languages and dialects. He never misses the obvious nor stumbles on the inventive. He has no private idiosyncratic preferences or dislikes. He never thinks laterally. He differs from all real people in one or more of these characteristics. A real worker in the field may never look at a piece of prior art—for example he may never look at the contents of a particular public library-or he may be put off because it is in a language he does not know. But the notional addressee is taken to have done so. This is a reflection of part of the policy underlying the law of obviousness. Anything which is obvious over what is available to the public cannot subsequently be the subject of valid patent protection even if, in practice, few would have bothered looking through the prior art or would have found the particular items relied upon. Patents are not granted for the discovery and wider dissemination of public material and what is obvious over it, but only for making new inventions. A worker who finds, is given or stumbles upon any piece of prior art must realise that that art and anything obvious over it cannot be monopolised by him and he is reassured that it cannot be monopolised by anyone else.
Laddie J. in Pfizer’s Patent [2001] F.S.R. 16 (at paragraph 62)
50.
The legal principles concerning the skilled addressee were set out in GlaxoSmithKline v Wyeth (PDF, 801 KB.pdf) [footnote 45]:
“(i) A patent specification is addressed to those likely to have a real and practical interest in the subject matter of the invention (which includes making it as well as putting it into practice).
(ii) The skilled addressee has practical knowledge and experience of the field in which the invention is intended to be applied. He/she reads the specification with the common general knowledge of persons skilled in the relevant art, and reads it knowing that its purpose is to disclose and claim an invention.
(iii) A patent may be addressed to a team of people with different skills. Each such addressee is unimaginative and has no inventive capacity.
(iv) Although the skilled person/team is a hypothetical construct, its composition and mind-set is found in reality. As Jacob L.J. said in Schlumberger v Electromagnetic Geoservices [2010] EWCA Civ 819: [2010] RPC 33 at paragraph 42:
“…The combined skills (and mindsets) of real research teams in the art is what matters when one is constructing the notional research team to whom the invention must be obvious is the patent is found to be invalid on this ground.””.
51.
As noted in GlaxoSmithKline v Wyeth [footnote 45] above the ‘person skilled in the art’ may be a multi-disciplinary team rather than a single individual; however care needs to be taken in selection of the members of that team. For example in Generics v Lundbeck [footnote 46] it was held that the skilled addressee for the development of an anti-depressant drug would include medicinal chemists, an analytical chemist and a clinician, but that process research chemists and pharmaceutical chemists would not be part of the team.
52.
In the chemical arts, where there is a vast library of available technical background much of which may be accessed by the relatively straightforward use of online searching tools, just what may be regarded as the common general knowledge can be problematic. Whilst the common general knowledge has been considered in numerous cases the statements made in Raychem Corp’s Patents [footnote 47] , Teva v AstraZeneca [footnote 48] and Angiotech v Conor[footnote 49] are particularly instructive. In Raychem Corp’s Patents the common general knowledge was deemed to be what the skilled person would know and take for granted as well as what he knows exists and would reach for as a matter of course.
The common general knowledge is the technical background of the notional man in the art against which the prior art must be considered. This is not limited to material he has memorised and has at the front of his mind. It includes all that material in the field he is working in which he knows exists, which he would refer to as a matter of course if he cannot remember it and which he understands is generally regarded as sufficiently reliable to use as a foundation for further work or to help understand the pleaded prior art. This does not mean that everything on the shelf which is capable of being referred to without difficulty is common general knowledge nor does it mean that every word in a common text book is either. In the case of standard textbooks, it is likely that all or most of the main text will be common general knowledge. In many cases common general knowledge will include or be reflected in readily available trade literature which a man in the art would be expected to have at his elbow and regard as basic reliable information.
Laddie J in Raychem Corp’s Patents [1998] RPC 31 (at page 40)
53.
In Teva v AstraZeneca [footnote 48] , Sales J. noted that in the age of the internet and digital databases of journal articles the guidance regarding common general knowledge needed to be adapted to reflect these advances. Whilst not all material available online constituted common general knowledge it was considered that material which the skilled addressee knows to be available online and which is generally accepted as a further basis for further action may form part of the common general knowledge.
The authorities indicate that CGK includes not just information directly in the mind of the notional skilled person, but such information as he would be able to locate by reference to well-known textbooks. This guidance needs to be adapted and kept appropriately up to date for the procedures for the dissemination of scientific knowledge in the age of the internet and digital databases of journal articles. Searches of such databases are part and parcel of the routine sharing of information in the scientific community and are an ordinary research technique. In my view, if there is sufficient basis (as here) in the background CGK relating to a particular issue to make it obvious to the unimaginative and uninventive skilled person that there is likely to be – not merely a speculative possibility that there may be – relevant published material bearing directly on that issue which would be identified by such a search, the relevant CGK will include material that would readily be identified by such a search.
Sales J. in Teva v AstraZeneca (asthma) [2014] EWHC 2873 (Pat), (2015) 142 BMLR 94 at paragraph 60
54.
In Angiotech v Conor it was noted that the common general knowledge also extends to that which the skilled person considers might work, and not just that which has been proven to work.
Common general knowledge’ is not formulaic - it is a term used in patent law to describe what the notional skilled person would know and take for granted. If the evidence shows that he knows people are looking at drug eluting stents as a way forward, then even if that has not been proved to work, it is nonetheless part of his mental equipment, not on the basis that he knows it will work but on the basis that it may.
Angiotech Pharmaceuticals Inc v Conor Medsystems Inc [2007] RPC 20 (Court of Appeal) at paragraph 18
Chiral Compounds: Enantiomers
55.
As noted under novelty above at paragraph 12, in most cases a single enantiomer is rendered obvious by prior disclosure of the racemate. An exception to this is where there is a technical prejudice such that the enantiomer cannot be straightforwardly prepared by standard resolution/separation techniques, even though the desirability of resolution/separation is known, as decided in Generics (UK) Limited v H Lundbeck A/S [footnote 9]. This practice was reaffirmed in Novartis AG v Generics (UK) Limited [footnote 50] (see also discussion of case under “Obvious to try” below) where it was held that the skilled team would consider resolution as a routine step. In this case Kitchen LJ stated:
The skilled team would consider that resolution of the racemate might bring practical benefits and would see resolution as a routine step.
Kitchen LJ in Novartis AG v Generics (UK) Limited (t/a Mylan) [2012] EWCA Civ 1623
Crystalline forms
56.
In general the mere provision of a compound in a crystalline form is not considered inventive (in the absence of a technical prejudice). In T 1555/127(PDF,438 KB) [footnote 7] the Board commented that “the mere provision of a crystalline form is not regarded as involving an inventive step. Investigation of whether active compounds are prone to crystalline transformation and characterisation of such crystalline forms is routine practice in the pharmaceutical industry.” A similar conclusion was reached in the earlier decision T 0777/08 (PDF, 250 KB). [footnote 51] Thus where the prior art teaches the existence of an amorphous (or undisclosed) form of the compound then the provision of a crystalline polymorphic form is unlikely to be inventive without an unexpected technical effect. In T 777/08 (PDF, 250 KB) “improved filterability and drying characteristics” were found not to represent such an effect as these characteristics would be expected of crystalline forms in general.
Obvious to try
57.
The classic statement regarding “obvious to try” is that given in Johns-Manville Corporation’s Patent (PDF, 1,743 KB) [footnote 52]. It was held that where a skilled worker in a particular field could be expected to know of a use of a material to achieve a certain result in that field, an invention which is concerned with the use of that material to achieve the same result in a part of that field in which it had not yet been used is obvious if a person versed in the art would assess the likelihood of success as sufficient to warrant a trial. Following later decisions, some of which are discussed below, the application of the “obvious to try” question has become a contentious issue, particularly in the chemical and pharmaceutical arts, however the fundamental question when assessing such cases should always be is it obvious? [footnote 50]
58.
In Johns-Manville Corporation’s Patent (PDF,1,743 KB) [footnote 52] the use of particular flocculating agents in asbestos cement manufacturing was considered. It was held that, filtration processes being common to many industries, the two cited documents were both likely to be read by those concerned with the asbestos cement industry despite the fact that they were addressed primarily to the mining and paper industries respectively. Such readers would have realised that here was a newly-introduced flocculating agent which it was well worth trying out in their filtration process.
I think that “would be” puts it too high if it postulates prior certainty of success before actually testing the polymers in the filtration process; it is enough that the person versed in the art would assess the likelihood of success as sufficient to warrant an actual trial.
Johns-Manville Corporation’s Patent, 1967, RPC 479
59.
In Johns-Manville Corporation’s Patent (PDF,1,743 KB) [footnote 52], all that was deemed necessary to render the invention obvious was the fact that it was obvious to try, and once tried success could be achieved without difficulty. By contrast, later decisions including Omnipharm Limited v Merial have been decided on the basis that there must be an expectation of success to render an invention non-inventive in this fashion. In Omnipharm Limited v Merial [footnote 53] this was considered in relation to a “spot on” formulation for the treatment of fleas in pets (a “spot-on” formulation being one for localised application to the animal’s coat, after which the composition spreads over the animal’s whole body by non-systemic mechanisms). The issue to be decided was whether the claim was obvious in light of prior art “spray on” formulations comprising the same active ingredient. Floyd J held that whilst it would be obvious to try and develop a “spot on” formulation, the skilled team would have no common general knowledge basis on which to make a prediction that the formulation would work (the mechanisms of action being poorly understood at the priority date) and as such the skilled person would not have had a sufficient expectation of success to render the invention obvious.
60.
The Judge in Omnipharm Limited v Merial referred to the Court of Appeal decision in Saint-Gobain PAM SA v Fusion Provida Ltd and Electrosteel Castings Ltd [footnote 54]. Here the cited prior art pointed to the possibility that using a Zn/Al alloy as a coating for a cast iron pipe to be buried in soil might be beneficial by showing results for this alloy as a coating for buried steel plates. It was not however possible for the skilled person to predict success and so the invention was deemed to be non-obvious (note however paragraph 65 below).
the ‘obvious to try’ test really only works where it is more-or-less self-evident that what is being tested ought to work.
Jacob LJ in Saint-Gobain PAM SA v Fusion Provida Ltd and Electrosteel Castings Ltd [2005] EWCA Civ 177, [2005] IP & T 880
61.
Therefore, in general, an invention can only be said to be ‘obvious to try’ if there is a reasonable expectation of success. Furthermore the bar set for an inventive step to be acknowledged is not changed according to the perceived level of support within the application/patent in suit provided that the disclosure of the patent specification is sufficient to make the invention plausible. In Conor Medsystems Inc v Angiotech Pharmaceuticals Inc. the House of Lords [footnote 55] rejected the lower courts’ ruling [footnote 56] [footnote 49], that the contribution to the art made by the specification had to be assessed in order to decide whether it was sufficient to show that something was an obvious candidate for testing without any expectation of success; or whether it was necessary to show that the skilled person must have had an expectation of success sufficient to induce him to use it in practice. Whilst the House of Lords accepted that the absence of any evidence to support a speculative claim could lead to an objection of lack of support or insufficiency (quoting the decision in Prendergast’s Applications) [footnote 57], they held that this requirement should not be confused with the requirement for inventiveness (see also the Manual of Patent Practice 3.87 and the Examination Guidelines for Patent Applications relating to Medical Inventions).
…there is in my opinion no reason as a matter of principle why, if a specification passes the threshold test of disclosing enough to make the invention plausible, the question of obviousness should be subject to a different test according to the amount of evidence which the patentee presents to justify a conclusion that his patent will work.’
Lord Hoffmann in Conor Medsystems v Angiotech Pharmaceuticals [2008] RPC 28 (at paragraph 37)
62.
In MedImmune Ltd v Novartis Pharmaceuticals UK Ltd [footnote 58] Kitchin LJ summarised the courts’ then current position on obvious to try (see paragraphs 90-95 of the decision), including the often used enquiry of whether it was obvious to pursue a particular approach with a reasonable or fair expectation of success as opposed to a hope to succeed, before stating that “ultimately the court has to evaluate all the relevant circumstances in order to answer a single and relatively simple question of fact: was it obvious to the skilled but unimaginative addressee to make a product or carry out a process falling within the claim.”
63.
A pharmaceutical example of an obvious to try decision is Novartis AG v Generics (UK) Ltd (see also paragraph 55 above). This decision concerned the ( ) enantiomer of N-ethyl-3-[(1-dimethylamino)ethyl]-N-methylphenyl-carbamate for the treatment of Alzheimer’s disease. The racemate (RA7) had previously been disclosed in two earlier publications as one of a number of compounds proposed for the treatment of Alzheimer’s disease. The issues to be decided were: whether it would have been obvious to select RA7 for further development from the compounds listed in the prior art; would it have then been obvious to resolve it; and lastly would it have been obvious to use the (-)-enantiomer as a pharmaceutical in the treatment of Alzheimer’s disease. Floyd J concluded that: there was nothing inventive in deciding to resolve and test RA7 to see if there were advantages or disadvantages associated with one of its enantiomers; and a pharmaceutical composition for the treatment of Alzheimer’s disease comprising the (-)-enantiomer was conceptually obvious and thus held that the patent was invalid.
64.
The decision in the lower court was appealed; it being contended that in using the “obvious to try” test there had been a failure to consider whether the skilled team would have had a reasonable expectation that the (-)-enantiomer would successfully treat Alzheimer’s disease, and that the “obvious to try” test was not applicable as it only applies to cases where it is more or less self-evident that what is being tested should work. The Court of Appeal considered the approach taken by Floyd J with the principles set out in MedImmune v Novartis [footnote 58] and, having reiterated that:
It cannot be said too often that the statutory question is: was the invention obvious at the priority date? It is not: was it obvious to try?
Kitchin LJ in Novartis AG v Generics (UK) Limited (Mylan) [2012] EWCA Civ 1623
found that the judge directed himself correctly as to the law and in a manner which was entirely consistent with the principles explained by the court in MedImmune and dismissed the appeal.
65.
The Court of Appeal in Novartis AG v Generics (UK) Ltd [footnote 50] also rejected the argument that the court can only make a finding of obviousness, when considering whether it is obvious to try a particular route, where it is manifest that that route ought to work:
…I reject the submission that the court can only make a finding of obviousness where it is manifest that a test ought to work. That would be to impose a straightjacket upon the assessment of obviousness which is not warranted by the statutory test and would, for example, preclude a finding of obviousness in a case where the results of an entirely routine test are unpredictable….
Kitchin LJ in Novartis AG v Generics (UK) Limited (Mylan) [2012] EWCA Civ 1623
66.
In Brugger and Others v Medic-Aid Ltd [footnote 59] Laddie J considered the issue of multiple possible avenues of research.
If a particular route is an obvious one to try, it is not rendered any less obvious from a technical point of view merely because there are a number, and perhaps a large number, of other obvious routes as well. If a number of obvious routes exist it is more or less inevitable that a skilled worker will try some before others….There is no rule of law or logic which says that only the option which is likely to be tried first or second is to be treated as obvious for the purpose of patent legislation.
Brugger and others v Medic-Aid Ltd [1996] RPC 635 (at page 661)
67.
However this should be read in the context of the decision in Saint-Gobain PAM SA v Fusion Provida Ltd and Electrosteel Castings Ltd [footnote 54], discussed above, as there it was held that the “[m]ere possible inclusion of something within a research programme on the basis you will find out more and something might turn up is not enough to show obviousness. If it were otherwise there would be few inventions that were patentable.”
68.
In Bristol-Myers Squibb Co v Baker Norton Pharmaceuticals Inc., [footnote 60] Jacob J held that an effect which was revealed by following the obvious course of action did not make the action non-obvious. It is wrong to ask whether you would have predicted the effect. In Pfizer Ltd’s Patent44 Laddie J stated that “whether something is obvious to try depends to a large extent on balancing the expected rewards if there is success against the size of the risk of failure”.
69.
In the chemical field, obvious to try objections are most frequently encountered in situations where an alternative set of reagents/reaction conditions may be used to achieve the same result with some expectation of either an improvement or other advantage in trying the alternative conditions. An important aspect of any obvious to try argument is that the means of enablement for the alternative (material, compound etc) must also be obvious. Thus, whilst it may be obvious to try to make a chemical compound, the claim to that compound will only be obvious if a method for its preparation is also obvious. [footnote 61]
Thus for a claim to the product to be held obvious the skilled man must not only envisage 4,6 blocked G5-p-NP to be a product, but also be able to obtain it or produce it without any step or thought that was not obvious.
Boehringer Mannheim v Genzyme [1993] FSR 716 (at page 726)
70.
In Pharmacia v Merck Aldous LJ [footnote 62] affirmed the view of Pumfrey J in the Patents Court that it was also obvious to investigate analogues (in this instance regioisomers of known Cox II inhibitors as anti-inflammatory agents - see structures below) of known pharmaceutically active compounds to determine their structure/activity relationship.
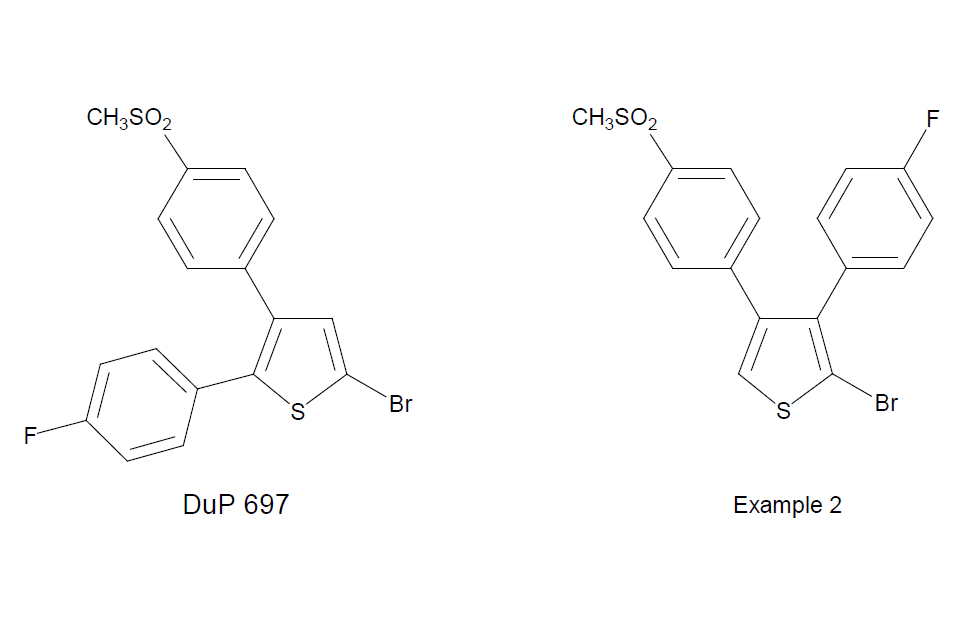
Structurally, there is a clear similarity between DuP 697 and its close 3,4 disubstituted analogues. It is a matter of reasonable prediction that they will have similar activity. I think that a medical chemist wishing to investigate the structure/activity relationship of DuP 697 would think of making its 3,4-diaryl analogues, with a view to seeing whether they are active. I also think that confronted with DuP 697 and required to develop a novel compound of similar activity, the 3,4-diaryl substitution is one of the first things which would occur to the medical chemist …. All the evidence gave me the clear picture that the 3,4 diaryl compounds were obvious to try for any skilled person knowing of DuP 697.
Pharmacia v Merck [2002] RPC 41 (at paragraph 141)
Plausibility and inventive step
71.
In the context of obviousness, it is clear from Hoffmann L.J.’s treatment of plausibility in Conor v Angiotech that, if it is considered at all, it is only as a first-step test which must be passed before going on to consider obviousness according to established principles. Thus this threshold test does not remove the need to apply the structure established in Windsurfing/Pozzoli and other tests including obvious-to-try (see above) and workshop variations. As noted earlier it is Office practice to consider this threshold under sufficiency - not least because the Windsurfing-Pozzoli analysis must necessarily be conducted on the claims rather than on a vague paraphrase based on the extent of the disclosure in the descripton. [footnote 55] For further guidance on selection inventions see paragraph 77.
Inventive step-sufficiency squeeze arguments and plausibility
72.
Carr J. provided guidance regarding the relationship between plausibility, sufficiency and obviousness in Actavis v Eli Lilly (587 KB). [footnote 36] The case concerned Lilly’s patent claiming the use of tamoxetine for the manufacture of medicament for treating attention-deficit hyperactivity disorder and validity was challenged by Actavis on the basis of obviousness and insufficiency. The patent contained no evidence to support the therapeutic efficacy claimed and Actavis sought to establish a squeeze-argument between the grounds of obviousness and sufficiency based on a lack of plausibility. Actavis argued that if it was held that the patent did contain an underlying principle to support the claimed therapeutic efficacy then this principle must have been acquired from the prior art. If Lilly argued that there was no reasonable expectation of success regarding the therapeutic effect from the prior art then the absence of data within the patent would render the invention implausible and consequently insufficient.
73.
As noted above, in general an invention can only be deemed ‘obvious to try’ if there is a reasonable expectation of success (see MedImmune v Novartis). [footnote 58] For Actavis to succeed in their argument they had to establish that the assessment of plausibility is the same as that for expectation of success. Actavis argued that, as with obviousness, plausibility requires a reasonable expectation of success that the invention would work. Lilly countered this by arguing that the threshold for plausibility was lower than that for obviousness. In his judgment Carr J. held that the standard for assessment of plausibility is not the same as the standard for assessment of expectation of success in the context of inventive step:
In my judgement, the policy considerations underlying plausibility for sufficiency are different from those underlying fair expectation of success for obviousness, which indicates that the standard for the assessment of plausibility is not the same as assessment for obviousness. For obviousness, a fair expectation of success is required because, in an empirical art, many routes may be obvious to try, without any real idea of whether they will work. The denial of patent protection based upon the “obvious to try” criterion alone would provide insufficient incentive for research and development in, for example, pharmaceuticals and biotechnology, and would lead to the conclusion that a research program of uncertain outcome would deprive a patent of inventive step. The reason why the court requires that the invention of a patent should be plausible is different. It is to exclude speculative patents, based on mere assertion, where there is no real reason to suppose that the assertion is true.
The cases on which Lilly relies (to which I have referred above) establish that the test of plausibility is a threshold test which is satisfied by a disclosure which is “credible”, as opposed to speculative. That disclosure may subsequently be confirmed or refuted by further evidence obtained subsequent to the priority date. If it subsequently shown that the invention does not work with substantially all of the products or methods falling within the scope of the claim then the scope of the monopoly will exceed the technical contribution and the patent will be invalid. This indicates why plausibility is only a threshold test. A plausible invention may nonetheless be shown to be insufficient. In my judgement the standard for assessment of plausibility is not the same [as] the standard for assessment of expectation of success in the context of obviousness.
Actavis Group PC EHF & Anr v Eli Lilly and Company [2015] EWHC 3294 (Pat) at paragraphs 177 and 178
74.
Carr J. rejected Actavis’s squeeze-argument and held that the claims were plausible and inventive over the prior art.
Agrevo Obviousness
75.
It is not Office practice to follow the problem-and-solution approach when assessing obviousness and in such situations Office practice is to deal with Agrevo obviousness under sufficiency. However the UK courts have now considered this line of argument and thus it is appropriate to consider it here.
76.
Agrevo obviousness arises when the technical effect is not made plausible for substantially all that is encompassed by the claim and as such the technical effect cannot form part of the technical problem against which obviousness is to be decided. In Idenix Pharmaceuticals Inc. v Gilead Sciences Inc., [footnote 63] Arnold J. considered application of the EPO Board of Appeal decision of AGREVO (T 939/92), [footnote 37] for assessing obviousness using the problem-and-solution approach. The case concerned compounds for the treatment of Flaviviridae, including hepatitis C. Interestingly, although the main claim was defined as a compound claim, Arnold J. considered that “…so far as the compound claims are concerned, inventive step should be assessed not on the basis that they are pure compound claims, but rather on the basis that they are claims to compounds which have anti-Flaviviridae activity. If it were otherwise, these claims would lack inventive step on the basis that the only technical problem they solved would be the provision of additional or alternative nucleoside analogues.” (paragraph 445). In light of Idenix’s expert evidence and lack of experimental data within the patent, Arnold J. held that it was not plausible that substantially all of the claimed compounds would have the necessary anti-Flaviviridae activity. A key point in his reasoning was that the patent did not contain any evidence or rationale to suggest that the claimed compounds had the anti-Flaviviridae activity only that “…the nucleosides of the invention “may inhibit Flaviviridae activity” and “can be screened for such activity using known assays…” (paragraph 458). Arnold J. held that the claims as granted, or proposed to be amended, lacked an inventive step on the grounds that the claimed invention did not make a technical contribution to the art reasoning that “… it covers compounds which the skilled team would not have considered plausible had anti-Flaviviridae activity and which therefore did not plausibly solve the technical problem of providing compounds which did have such activity.”.
Selection inventions
77.
Although there is no inventive step if it is clear from the prior art that taking a particular step is likely to lead to success, there may be invention if that step is one of many courses possible, and there is no reason to infer from the prior art that this one is more likely than the others to be profitable. Selection inventions are particularly relevant when citing a prior art disclosure of a broad class (e.g. a Markush structure) against a claim to a narrow sub-class (e.g. a single compound or a relatively small number of compounds).
78.
The previous Office practice on selection inventions was based on the criteria (‘the I G rules’) set out in I G Farbenindustrie AG’s Patent, [footnote 31] but has been changed following the decision of the UK Court of Appeal in Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly & Co Ltd. [footnote 19] This utilised an approach based on EPO Board of Appeal decisions (in particular [T 939/92 AGREVO/Triazoles][footnote 37] and T 133/01 WYETH) [footnote 64] wherein the contribution of the application was considered and a selection would be regarded as obvious if it had made no real technical advance.
…it regards what can fairly be regarded as a mere arbitrary selection from a class as obvious. If there is no more than an arbitrary selection then there is simply no technical contribution provided by the patentee.
Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly & Co Ltd [2010] RPC 9 (at paragraph 44)
79.
When faced with claims that may relate to a selection invention the prima facie inventive step objection should be raised using (implicitly or explicitly as appropriate) the Windsurfing/Pozzoli approach, [footnote 43] unless the selection is so clear-cut as to make this unnecessary (such situations may include chemical process or polymer cases where the nature of the selection is both described with reference to the prior art and clearly demonstrated in the examples). If the applicant maintains (or it is clear from the specification) that the inventiveness may lie in a selection invention, then the approach used by the Court of Appeal in Dr Reddy’s Laboratories v Eli Lilly. [footnote 19] should be followed. In such cases, the question to be asked is whether the invention makes a hitherto unknown technical contribution or is merely an arbitrary selection. If it is merely an arbitrary selection then the invention is obvious.
80.
Whilst it may be straightforward to establish whether the selection is arbitrary in nature when considering the selection of a single substance from a larger class as in Dr Reddy’s Laboratories v Eli Lilly, [footnote 19] the nature of the selection will frequently not be so readily determined e.g. when a sub-range is being selected from a larger range. In Generics [UK] Ltd v Yeda Research and Development Co. Ltd [footnote 65]. the Court of Appeal considered the law regarding selection inventions, with reference to Dr Reddy’s Laboratories v Eli Lilly, [footnote 19] T 939/92 AGREVO/Triazoles, [footnote 37] and Conor Medsystems v Angiotech Pharmaceuticals. [footnote 55] The position following the judgment in Generics v Yeda [footnote 65] is as follows:
i) Article 56 of the EPC (equivalent to s.3 of the Act) is in part based on the underlying principle that the scope of the patent monopoly must be justified by the patentee’s contribution to the art;
ii) If the alleged contribution is a technical effect which is not common to substantially everything covered by a claim, it cannot be used for the purposes of judging obviousness;
iii) In such circumstances the claim must either be restricted to the subject matter which makes the technical contribution, or a different contribution common to the whole claim must be found;
iv) A selection from the prior art which is purely arbitrary and cannot be justified by some useful technical property is likely to be held to be obvious because it does not make a real technical advance;
v) A technical effect which is not rendered plausible by the patent specification may not be taken into account in assessing inventive step;
vi) Later evidence may be cited to support a technical effect made plausible by the specification;
vii) Provided the technical effect is made plausible, no further proof of the existence of the effect is to be demanded of the specification before judging obviousness by reference to the technical effect put forward. [footnote 66]
81.
The “hitherto unknown technical effect” (i.e. advantage gained or disadvantage avoided) relied upon to justify a selection invention should be clearly identified or otherwise made plausible (e.g. discernible from tests provided in the application), in the specification at the time of filing (see also T 1329/04 Johns Hopkins University School of Medicine/Growth Differentiation Factor [footnote 38]). Later-filed evidence may be used to provide support for the presence of such an effect or the fact that it is common to everything claimed, but unexpected bonus effects not described in the specification cannot form the basis of a valid claim to a selection invention (see Glaxo Group Ltd’s Patent [footnote 67]) If there is no statement of advantage in the specification at the time of filing, it may not be added later, since, as stated by Jacob J. in Richardson-Vicks Inc.’s Patent, [footnote 68] in the context of synergy, whether or not the advantage was demonstrated “by experiments conducted after the date of the patent cannot help show obviousness or non-obviousness… and it would be quite wrong for later-acquired knowledge to be used to justify the amended claim.”
82.
The judgment in Generics [UK] Ltd v Yeda Research and Development Co. Ltd. [footnote 65] also addressed the question of what happens if the technical property or effect made plausible by the specification does not exist in fact. The lower court had held that since later evidence cannot be used to support a technical effect not indicated in the specification, neither can it be used to refute such an effect. The Court of Appeal held however that in considering later evidence on this issue one is not judging the obviousness of the invention by reference to later evidence; one is simply defining by evidence what the invention is. The Court allowed the admission of later evidence which, according to the plaintiff, showed that the composition as claimed did not demonstrate the relied upon technical effect. The Court however found that the evidence provided did not prove the absence of the technical effect and rejected the appeal.
83.
The requirement for “an unexpected technical effect” to support a selection invention is consistent with the judgment in Conor Medsystems v Angiotech Pharmaceuticals. [footnote 55] Conor Medsystems v Angiotech Pharmaceuticals requires that support and inventive step should not normally be conflated as the test for inventiveness should not change provided the invention is plausible. Selection inventions cover the very situation where the plausibility of the invention is at issue; the selection invention only being plausible if the “unexpected technical effect” is clearly identified or identifiable as this is what is being relied upon to distinguish the selection from the prior art and so establish the inventive step of this invention.
84.
EPO Technical Board of Appeal decision T 181/82 [footnote 69] suggests that where comparative tests are submitted as evidence of an unexpected technical effect, there must be the closest possible structural approximation between the prior art compound tested and the subject-matter of the invention; and that only known substances - not notionally described ones - qualify for use in comparisons of compounds.
85.
The technical significance of the parameters by which the product or process is selected should be considered. Where unusual parameters are used in a claim it may be difficult to prove whether or not the prior art would have inevitably exhibited those parameters (see “Clarity and construction of claims” section below). If arbitrary parameters are used they are considered to be non-technical and may be ignored in the assessment of obviousness (and by extension novelty).[footnote 47] [footnote 70]
Collocations and synergy
….before you can apply s.3 and ask whether the invention involves an inventive step, you first have to decide whether you are dealing with one invention or two or more inventions. Two inventions do not become one invention because they are included in the same hardware. A compact motor car may contain many inventions, each operating independently of each other but all designed to contribute to the overall goal of having a compact car. This does not make the car a single invention.
Sabaf SpA v MFI Furniture Centres Ltd [2005] RPC 10 (at paragraph 24)(House of Lords)
86.
An inventive step might be provided by a specific combination of elements of an invention, such as a specific combination of two compounds or polymers in a composition. The House of Lords in Sabaf [footnote 71] considered the inventive step of an invention that had a number of different components. In his judgement, Lord Hoffman referred to the EPO Examination Guidelines relating to the issue of combination versus juxtaposition or aggregation when considering inventive step. The EPO Guidelines [footnote 72] state that “…where the claim is merely an aggregation or juxtaposition of features and not a true combination, it is enough to show that the individual features are obvious to prove that the aggregation of features does not involve an inventive step. A set of technical features is regarded as a combination of features if the functional interaction between the features achieves a combined technical effect which is different from, e.g. greater than, the sum of technical effects of the individual features.” In other words, if each component of the invention interacts with each other, and thus there is synergy, then they relate to a single inventive concept having a combined effect. However, if each component performs its own normal function independently of any of the others then each relates to a separate inventive concept.
87.
This was considered in BL O/220/13 which centred on two applications for vitamin supplement compositions, the first for bone health maintenance and the second for postnatal health and lactation. The hearing officer considered that, for such compositions to be inventive, there had to be some degree of synergy between the constituents. Neither of the applications, however, provided any suggestion that the constituents interacted with each other in any way. It was considered that although there was an inevitable degree of interaction when present in the human body, this was quite unintentional and incidental to the operation of the composition. Assessment of inventive step was therefore conducted on the basis of each of the constituents individually. As each constituent was well known in the art for use in dietary supplements, the invention was considered to be an obvious collocation and both applications were refused for lack of inventive step.
88.
Synergistic effects are most often encountered in the chemical arts when active compounds are combined in pharmaceutical formulations; however they also appear in other applied chemistry fields. For some inventions, the synergistic effect may not be clear cut and thus careful consideration of the examples will be necessary. Indeed collocation arguments may not be appropriate in some multi-component compositions, for example toiletries products where, despite the fact that each component is essentially acting in its normal fashion, its presence in the composition may require ‘tuning’ of the proportions of the other components to counter any undesirable properties of the component. UK case law currently states that the synergistic effect must be plausible on the basis of the application as filed. [footnote 67] [footnote 68]
Compositional cases
89.
Having noted above that collocation arguments may not be appropriate when assessing the inventiveness of claims to multi-component compositions, it is appropriate to give some thought to the search and examination of compositional claims. When assessing if a composition claim may be obvious, it is important to consider the nature of the disclosure in the prior art. In general, the mere fact that each of the required components for a particular compositional claim are present in lists of possible ingredients within one document should not be regarded as destroying the inventiveness of that composition. Instead search and examination should generally concentrate on those prior art disclosures where the exemplified compositions have much in common with the claimed compositions, but differ in only one or a few respects. The extent to which the prior art examples and the claimed compositions differ and yet a valid inventiveness argument can be made will depend in large part on the art. In compositional cases, what forms part of the common general knowledge or would be regarded as routine laboratory modification should be considered. For instance, a prior art example composition sharing the bulk of the required ingredients, but possessing the missing ingredients in a short preferred list of obvious replacements for a given example ingredient, or a prior art example with all of the required components, but where not all of the components are present in the required proportions, should generally be used as the basis of obviousness objections. Equally a prior composition, where the missing components are merely standard additives for the end use, may form the basis of a strong inventive step argument (e.g. carbon black added as a UV stabiliser). The applicant may then put forward arguments with respect to whether the replacements or alteration of proportions required is indeed inventive.
Generic overlap (Markush claims and prior art Markush structures)
90.
When a claim comprises a Markush structure which is of overlapping scope to a Markush structure contained in the prior art then it is Office practice, in contrast to that of the EPO, to object to the claim as lacking an inventive step rather than novelty (see “Prior disclosure: individualised description and Markush structures” at paragraph 18 above)). Thus the Markush structures are treated in effect as defining classes of compounds. Rather than determining the extent to which one group might be considered to be coterminous with another (and thus whether novelty is appropriate) it has been deemed pragmatic to simply object under obviousness. Nevertheless, the strength of this objection will in part depend on matters such as the intended use (whether explicitly mentioned in the claim being examined or not) and on the extent of the overlap. (For example, do the two Markush structures share a common core or in the case of polymers do they share the same groups pendant from the polymer backbone?). In situations where there is a clear, supported selection invention (see paragraph 77 onwards)) then an obviousness objection need not be pursued.
Industrial application
91.
The wording of section 1(1)(c) requires that an invention must be “capable of” industrial application. Section 4(1) further states that an invention is capable of industrial application if it “can be made or used in any kind of industry”. In [Chiron Corp., the Court of Appeal observed that section 4(1) is not satisfied if the product made is useless. [footnote 73]
….the sections require that the invention can be made or used “in any kind of industry” so as to be “capable” or “susceptible of industrial application”…..But industry does not exist in that sense to make or use that which is useless for any known purpose.”
Chiron Corp v Murex Diagnostics Ltd [1996] RPC 535 (at page 607)(Court of Appeal)
92.
It is therefore necessary to consider whether the invention claimed has a useful purpose, and whether the specification identifies any practical way of exploiting it. It is not the purpose of a patent to reserve an unexplored field of research for an applicant. [footnote 74]
Assessing industrial application
93.
Determining if a chemical invention is capable of industrial application (i.e. has a useful purpose) can be difficult because the industrial application of a compound may not apparent from the invention itself. Thus, the question arises what needs to be shown to establish that a chemical invention is capable of industrial application.
94.
In recent years, the tests for industrial application in both the Office and the EPO have moved away from the “specific, substantial, and credible” utility requirement derived from US practice. Recent UK and EPO case law has considered whether the proposed industrial application is “plausible”. [footnote 75] In general where some industrial application is suggested, but there is some doubt over the “plausibility” of that utility then the applicant may in some circumstances provide additional information demonstrating that the stated utility is indeed plausible. (This of course does not apply where the stated industrial application is covered by section 4A of the Act and the application relies on the use for novelty/inventiveness. See the Examination Guidelines for Patent Applications relating to Medical Inventions.
Plurality
95.
The fact that the inventions defined in independent claims may be directed to solving the same problem or to implementing the same idea, or that separately claimed processes may lead to the same product, may not be sufficient in itself to confer unity of invention. In particular the fact that a class of chemical intermediates has been prepared solely in order to be converted to particular products may not demonstrate that there is a single inventive concept linking claims to the intermediates and the products. The EPO Technical Board of Appeal held in Decision T 35/87 [footnote 76] that it is necessary for unity of invention between intermediates and end-products that groups of intermediates prepared and oriented towards the end-products be closely technically interconnected with the latter by sharing an essential structural element. Therefore, if intermediate and final products include a common structure which is both novel and inventive then claims to the products can relate to a single inventive concept even though separate searches for the intermediates and products might be necessary.
96.
Where an applicant has discovered a useful property or activity in a group/class of chemically related compounds, some of which are known, claims to the new use (subject to the provisions of section 4A(1)), to compositions containing the compounds for such use, and to any of the compounds that are novel per se and to their method of preparation, may be considered to form a single inventive concept provided that all of the compounds, whether novel or known, possess the common characterising property giving rise to the use. This in turn assumes that the combination of the particular use and class of compounds is itself novel and inventive.
97.
Where there is lack of unity of invention, it is the first invention encountered in the claims that must form the basis of the search. [footnote 77] This applies not only where there are independent claims forming separate inventions, but also within claims when they relate to a number of different inventive concepts (e.g. several divergent Markush formulae in one claim).
Sufficiency
98.
Section 14(3) requires that the specification must be clear and complete enough for the invention to be performed by a person skilled in the art. Identification of the skilled person is straightforward. For most purposes this uninventive, but technically competent person is considered as having the same level of skill as for the purpose of assessing inventive step (See paragraph 49 onwards)). In addition, the skilled person is someone seeking to make the patent work and may use the common general knowledge at the time of filing the application to supplement the information contained within the specification.
99.
The worked examples in a specification can omit certain basic steps, if these can be derived by routine trial and error, and can even include obvious mistakes if the skilled person could be expected to spot and correct them. [footnote 78] However, the experimentation must not require any inventive skill, [footnote 79]. For instance, a third party may be expected to investigate a necessary stirring speed when presented with a recipe requiring stirring. However, where a recipe is nebulous, or where it also depends on one or more parameters and the methods of achieving these parameters is not disclosed, then there is an undue burden on the skilled person. [footnote 80]
100.
There is no precise definition of what constitutes an ‘undue burden’, but it can be equated with such things as the need for more than routine (i.e. excessive) experimentation; costly or burdensome field trials; or where the teaching of a disclosure is no more than the starting point of a research programme. In each case this is a question of fact, depending on the nature of the invention and the disclosure of the specification (see the Manual of Patent Practice 14.85-14.88).
101.
Case law in this area has developed such that insufficiency is often characterised as falling into one or more of the following categories: (i) classical insufficiency; (ii) insufficiency due to ambiguity; and (iii) ‘Biogen’ insufficiency, or ‘excessive claim breadth’. Although these are non-statutory terms, which may in practice overlap to some extent, it is helpful to make it clear to the applicant which is at issue when making a sufficiency objection. The three types of insufficiency are discussed below.
(i) Classical insufficiency
102.
Classical insufficiency arises when the disclosure of the invention is not complete enough for the claim to be worked at all by a person skilled in the art, i.e. there is no enabling disclosure that allows the invention to be worked to any extent. Owing to the prohibition on adding new subject matter, such an objection is potentially fatal to the application. In a chemical context, an example of this might be where the invention relates to a new compound, but there is no, or not enough, disclosure of how to make the new compound, and its manufacture would not be apparent to the skilled person from common general knowledge. Other examples might be a claim to a material having a particular structural property, such as porosity, or crystal structure, where there is no disclosure of how this property might be achieved in practice.
103.
The applicant does not have to disclose the best mode of carrying out the invention, nor does it have to disclose preferred embodiments: the application needs only to provide the fundamental technical requirements. For instance, a useful perfecting step in making a compound does not have to be disclosed if the imperfectly produced compound can still be used for the application’s purpose. [footnote 81]
(ii) Insufficiency due to ambiguity
104.
A second form of insufficiency arises when the disclosure is so ambiguous as to make it impossible to know whether one had worked the invention or not. This was distinguished from lack of clarity in Kirin Amgen. [footnote 24] The relevant claim required the rEPO of the invention to have a higher molecular weight compared to uEPO. However different uEPOs have different molecular weights and the description failed to teach which uEPO was the comparator. Lord Hoffman elaborated “In the present case, however, the choice of uEPO has nothing to do with making the invention work. It is simply a criterion against which one tests whether rEPO falls within the claims…All the skilled man can do is try and guess which uEPO the patentee had in mind and if the specification does not tell him, then it is insufficient.” Elsewhere in his judgement Lord Hoffman provided an artificial example of how lack of clarity can overstep the mark into insufficiency:
If the claim says that you must use an acid, and there is nothing in the specification or context to tell you which acid, and the invention will work with some acids but not with others but finding out which ones work will need extensive experiments, then that in my opinion is not merely lack of clarity; it is insufficiency. The lack of clarity does not merely create a fuzzy boundary between that which will work and that which will not. It makes it impossible to work the invention at all until one has found out what ingredient is needed.
Kirin-Amgen Inc & Ors v. Hoechst Marion Roussel Limited & Transkaryotic Therapies Inc, [2004] UKHL 46
105.
In Sandvik Intellectual Property AB v Kennametal UK Ltd, [footnote 82] a claim to a material having a particular texture coefficient was found to be insufficient due to ambiguity because calculating the value required reference to a value measured against a standard and there was no disclosure of which of the two widely used common standards was used by the patentee.
106.
In T1914/11,[footnote 83] it was found that a claim to a liquid formulation, which included a requirement that the formulation was ‘alkalized by buffering and/or pH regulation in such a way that upon administration to a subject the pH of the liquid in the oral cavity of the subject is transiently increased by about 0.3 to about 4 pH units’ was not only unclear, but was also insufficient. The Board held that in the absence of any test method and further teaching regarding the formulation, the problem faced by the skilled person was not only a lack of mathematical precision but also a real burden to realise and reproduce the invention.
(iii) Insufficiency due to excessive claim breadth (‘Biogen’ insufficiency)
107.
Whereas classical insufficiency involves considering whether the claimed invention is sufficiently enabled to any extent, insufficiency due to excessive claim breadth arises when the application contains sufficient disclosure for only part of the claim to be worked, but not its entire scope. Showing that the full scope of the claim can be worked is usually done by a combination of general disclosure and particular embodiments. In the case of a single compound, only one example will be needed, as held in Generics (UK) Limited & Others v H Lundbeck A/S, [footnote 9] since producing a compound for the first time entitles the applicant to a monopoly for that product regardless of others subsequently inventing new methods of producing it. If the claim is more complex, for instance being claimed as a generic class, or as a Markush formula, then it is likely that multiple examples will be required. An objection to excessive claim breadth can be overcome by suitable restriction of the claims. At the search stage, the examiner should consider whether it is necessary to search the full scope of a claim when the portion of the scope of the claim which is insufficiently supported must be removed anyway, though any restriction of the search should be clearly communicated to the applicant.
108.
This type of insufficiency is also called ‘Biogen insufficiency’, after the decision in Biogen Inc. v Medeva plc, [footnote 79] which established that the entire scope of the claim must be sufficiently disclosed. The House of Lords held that:
If the invention discloses a principle capable of general application, the claims may be in correspondingly general terms…On the other hand, if the claims include a number of discrete methods or products, the patentee must enable the invention to be performed in respect of each of them.
Thus if the patent has hit upon a new product which has a beneficial effect but cannot demonstrate that there is a common principle by which that effect will be shared by other products of the same class, he will be entitled to a patent for that product but not the class, even though some may subsequently turn out to have the same beneficial effect.
Lord Hoffmann in Biogen Inc v Medeva plc [1997] RPC 1 (House of Lords) (at pages 48-49)
Biogen insufficiency and Markush claims
109.
This principle in relation to a Markush claim was considered in Pharmacia Corporation and Others v Merck & Co Inc., [footnote 62] where it was held:
Where the claimed invention is to a class of compounds, the same principle applies and, as was made clear by the House of Lords in Biogen, is that the disclosure in the specification must enable the invention to be performed to the full extent of the monopoly claimed. Thus if the invention is a selection of certain compounds, in order to secure an advantage or avoid some disadvantage, not only must the specification contain sufficient information on how to make the compounds, it must also describe the advantage or how to avoid the disadvantage. Further the compounds monopolised by the claim must all have that advantage or avoid the disadvantage. The same principle applies where the claim is to a class of compounds. To be sufficient, the specification must identify the characteristics of the class and a method of manufacture. Further all the claimed compounds must in substance have the characteristics of the class.
Pharmacia v Merck [2002] RPC 775 (at paragraph 56)
Claims defined by function
110.
In American Home Products Corporation & Anor. v Novartis Pharmaceuticals UK Ltd & Anor [footnote 84]. the question of a common principle hinged on the function of the class, in this case immunosuppressant activity, where only rapamycin was shown in the patent as having this function. A claim attempting to extend this disclosure to derivatives of rapamycin as a class, when the specification contained no disclosure of any such derivatives, was held to be little more than a starting point for a research programme, and thus insufficient.
111.
The concept of sufficiency related to a class defined by a functional limitation applies not to only specific chemicals (e.g. a drug), but also to general materials. In T435/91 [footnote 85] , the Technical Board of Appeal considered a claim to a gel characterized as ‘wholly or predominantly in hexagonal crystal form’, and which listed various components of the gel, including a surfactant system, and an additive ‘capable of forcing the surfactant system into a hexagonal phase’. This functional definition of the additive was held to be insufficient as the claim encompassed all additives without giving any useful technical guidance as to how to obtain, with a reasonable expectation of success, further suitable additives which were not ‘hydrotropes’. In this case, amendment of the claim to specify that that the additive was a hydrotrope was considered enough to overcome the sufficiency objection.
112.
Care should be taken with respect to sufficiency when the applicant seeks to overcome an objection (whether to excessive claim breadth, novelty or inventive step) by inclusion of a functional limitation. In Novartis AG, Cibavision AG v Johnson & Johnson Medical Limited, [footnote 86] the court considered a claim to a contact lens formed from two unspecified polymers defined by parameters, including functional parameters. The judge stated:
…It follows, in my judgment, that a claim to a class of products said to possess a useful activity must be based upon the identification of a common principle which permits a reasonable prediction to be made that substantially all the claimed products do indeed share that activity. Further, it is not permissible to by-pass that requirement simply by adding a functional limitation which restricts the scope of the claim to all products which do have the relevant activity, that is to say all those which ‘work’. In the case of a claim limited by function, it must still be possible to perform the invention across the scope of the scope of the claim without undue effort. That will involve a question of degree and depend upon all the circumstances including the nature of the invention and the art in which it is made. Such circumstances may include a consideration of whether the claims embrace products other than those specifically described for achieving the claimed purpose and, if they do, what those other products may be and how easily they may be found or made; whether it is possible to make a reasonable prediction as to whether any particular product satisfies the requirements of the claims; and the nature and extent of any testing which must be carried out to confirm any such prediction.
Novartis AG, Cibavision AG v Johnson & Johnson Medical Limited [2009] EWHC 1671 (Pat) (at paragraph 244)
Plausibility/credibility with regard to sufficiency
113.
It must be possible to make a reasonable prediction from the information provided in the specification that the invention will work with substantially everything falling within its scope, in other words, it must be ‘plausible’ or ‘credible’ that the invention can be worked across the full extent of the claim. However, this is only a threshold requirement, and even if it passes this threshold, it may still subsequently be shown that the invention is insufficient. As was held in Idenix Pharmaceuticals Inc. v Gilead Sciences Inc. [2014] EWHC 3916 (Pat), [footnote 63] at paragraph 467:
For the reasons set out above, the court must undertake a two-stage enquiry. The first stage is to determine whether the disclosure of the Patent, read in the light of the common general knowledge of the skilled team, makes it plausible that the invention will work across the scope of the claim. If the disclosure does make it plausible, the second stage is to consider whether the later evidence establishes that in fact the invention cannot be performed across the scope of the claim without undue burden. In some cases, it is convenient to divide the second stage into two, first considering whether the invention can be performed without undue burden at all and then whether the claim is of excessive breadth.
114.
A similar two-step approach to sufficiency considering first plausibility and then whether there was an undue burden to working the claim across its full breadth was utilised in Eli Lilly v Janssen Alzheimer Immunotherapy. [footnote 87] For a general discussion of plausibility see paragraphs 40-46 above.
Section 14(5): support
Relationship to section 14(3)
115.
Section 14(5) support can overlap with that of section 14(3), but is a less serious flaw in the patent application, generally more easily overcome by amendment. Previous Office practice was to object to broad and speculative claims under section 14(5) as unsupported by the description. However, in clear cases where this arises, section 14(3) should be used instead of, or as well as, section 14(5), especially since insufficiency is a grounds for revocation under section 72(1)(c), whereas lack of support isn’t. It should also be noted that support has particular significance with regard to claims defined by medical use (see paragraph 118). [footnote 88] [footnote 57]
116.
Section 14(5) is more generally used where the scope of the claims is contradicted by the scope of the description, for instance where an example falls outside the scope of the claims.
117.
Support for ranges within the description may differ depending on their relevance. In a standard case, each range needs little more than a counterpart in the description and if this is absent then an objection should be raised under section 14(5). It should be noted that the range is considered as a single entity and combining the lower end from one range with the upper end of a different range is not allowed. [footnote 89]
Medical use claims
118.
It is well established in UK law that support, such as rudimentary tests, for second, and by analogy first, medical use claims must be present in the application as filed. [footnote 57] This is thoroughly covered in the Examination Guidelines for Patent Applications relating to Medical Inventions; however it is worth noting here that it is Office practice not to consider support for first and second medical use claims as a matter of practicality in applications where these claims have been included as subsidiary claims to a main claim or claims relating to a new compound, and the substance or composition claim is new, inventive and supported by the description.
Nevertheless, in cases where it transpires that the per se claims are not new and inventive and it appears that the claimed medical use(s) may be the only novel and inventive feature, a warning should be issued at search stage if such uses are not supported by evidence in the application as filed.
Selection inventions
119.
Selection inventions rely on the fact that the applicant has discovered within a previous invention a particular technical effect which was not disclosed in that previous invention. Support is generally required for establishing such, although this may in some circumstances be provided as later-filed evidence if the selection is identified in the application as filed (see discussion of selection inventions under inventive step above). In terms of ranges, simple assertions about the endpoints are acceptable provided that worked examples genuinely represent a cross-section of points in the range, and such examples in conjunction with comparative examples (or by contrast with the prior art) demonstrate the desired technical effect. Similarly, for Markush structures, there need to be multiple examples which demonstrate the technical effect claimed.
Reach through claims
120.
Reach through claims seek to protect things which may not have been identified by the applicant at the time of filing but which may be identified in the future by carrying out the applicant’s process. Thus the claims ‘reach through’ to things which the applicant has not yet identified. Such speculative claims differ from ‘product by process’ claims because the product of a process requires repetition of the process to obtain more product, whereas the subject of a ‘reach though’ claim does not. It follows that ‘reach through’ claims may even extend to known materials which are not modified in any way by the process used to identify them. Examples of such claims are those directed towards candidate compounds that are identified by the use of screening methods. Such compounds are generally only defined by their function e.g. as modulators of receptor X, and no relationship between this function and the structural features of the compounds is described. In the absence of any knowledge of any relationship, either from the specification or from common general knowledge, the skilled person would not know how to produce and use the compounds. Moreover, the skilled person would not know before undertaking the laborious task of performing the screening assay if any given compound would fall within the scope of the claim. It would require an undue burden of experimentation to screen undefined compounds for the desired activity. There will also be a lack of support where the function of the compounds identified is not specified. Consequently this type of claim ought not to be searched, and should be objected to at examination stage.
121.
There is currently no European or UK case law relating specifically to reach through claims. However, a US Federal Court of Appeal case considered the validity of reach though claims. [footnote 90] Claims to compounds identified in a screening assay, with no disclosure of what the compounds might be, were found to be invalid on the basis that they did not meet the written description requirements of a patent application, i.e. the claims were attempting to encompass subject matter that was not described within the specification. Whilst US case law has no bearing upon the application of UK patent law, the basis for objection to reach through claims by the Office would be the same, i.e. the scope of the claims extends beyond what has been disclosed in the description.
Exclusions under section 1(2)
122.
Patent applications in the chemical area can comprise subject matter that is excluded under section 1(2) of the Act, such as computer programs, mathematical models and methods for performing a mental act. These should be dealt with as outlined in the appropriate section of the Manual of Patent Practice see 1.07 onwards, however further consideration of discoveries is provided here due to their importance in chemical patent applications.
Discoveries
123.
Discoveries in pure chemistry are largely only a philosophical issue. Theoretically, it is impossible to say whether a new compound already existed in nature and the applicant has stumbled across a way of synthesising it, or whether the applicant has genuinely invented a new compound. However, the practice of allowing patents for new products per se has been recognised in UK law continuously since 1949 (and endorsed by Article 167 of the EPC).
124.
In cases of old compounds, newly found properties of those compounds are considered to be discoveries; however, the use of such materials does not constitute a discovery, even if, as established in Genentech Inc.’s Patent, the use is startlingly obvious. This approach was upheld by Kirin-Amgen v Hoechst Marion Roussel [footnote 91] in the context of extraction and isolation of genes with no previously recognised existence.
125.
Tate & Lyle Technology v Roquette Frères [footnote 34] discussed the claim: “The use of maltotriitol to modify or control the form of maltitol crystals”. The term “control” was construed as including the option of not changing the level of maltotriitol from that readily established in the prior art. Therefore, the claim was construed as merely explaining the science which caused known effects to happen in known processes and (as noted in the discussion of use claims above at paragraphs 32-33) was a discovery as such. The addition of the words “the use of” in this case merely changed the form of the claim, not the substance of the claim. It was noted that “it would have been possible to claim particular processes or products that took advantage of the discovery, for instance by claiming certain levels of concentration of maltotriitol within the syrup, or crystals produced with the aid of the discovery.”
Clarity and construction of claims
Claims defined by parameters
126.
Where the invention relates to a chemical compound it may be defined in a claim in various ways, e.g. by its chemical formula, or, exceptionally, by its parameters or as a product of a process. Definition of a chemical compound solely by its parameters should, as a general rule, be allowed only in those cases where the invention cannot be adequately defined in any other way, for example in certain polymer cases. In such cases however only parameters usual in the art should be employed to define the compound/polymer, since use of unusual parameters may disguise lack of novelty.
127.
The technical significance of the parameters by which the product or process is defined should be considered. Where unusual parameters are used in a claim it may be difficult to prove whether or not the prior art would have inevitably exhibited those parameters; such parameters may even be intended to be hard to understand. In Raychem Corp.’s Patents [footnote 47] it was held (at pp.46-47) that:
although it may not be obvious, in the common use of that word, to limit a claim by reference to some particular meaningless and arbitrary parameter, that had nothing to do with patentability. Patents are not given for skill in inventing technically meaningless parameters.
Product-by-process claims
128.
As described above (paragraph 24) under novelty, the House of Lords in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd held that ‘product-by-process’ claims (e.g. “Product X obtained by process Y”), should be construed as a claim to the product per se; this is irrespective of whether the term “obtained”, “obtainable”, “directly obtained” or an equivalent wording is used. A claim for a patentable product defined by its process of manufacture is only allowable if the product cannot satisfactorily be characterised by reference to its structure or composition; if the product can be defined by other means, an objection under clarity and/or conciseness should be raised.
Claims involving plant/animal extracts
129.
Extracts from plant or animal material are a particular class of inventions where product-by-process claims are likely to be acceptable (assuming that the nature of the material being extracted is not known or readily determined). In such cases, it is important that the nature of the extract is well defined. Factors to consider include the nature of the extraction solvent (this will have a large bearing on the compounds likely to be extracted, for example certain organic solvents may result in a great deal of organic material in the extract whereas use of water in extreme circumstances could yield an extract consisting essentially of water), the part of the plant/animal being extracted (e.g. root extracts are likely to have a different composition to seed, bark or leaf extracts) and the conditions employed (room temperature mixing or Soxhlet extraction over a period of days).
Process claims
130.
More generally, chemical “process” claims should define the starting material, the end product and also the means adopted for converting the one into the other. [footnote 92] The definition of a process in the claims with reference to terms such as condensation, polymerisation, esterification and sulfonation, or even by the use of the term “reacting”, is permissible provided the specification contains no reservations about the universality of the process.
Composition/alloy claims
131.
The extent to which the ingredients of a composition need to be specified in order adequately to define the invention depends greatly on the subject-matter concerned. Thus a claim to “a pharmaceutical composition containing compound X together with a diluent or carrier” is allowable, X being a medically active compound which characterises the composition, and the diluent or carrier being any material suitable for that purpose and being selectable by knowledge of the art or by non-inventive experiment. In the alloys field, a sufficient number of the constituents should be specified such that the claim is not speculative and is adequately supported by the disclosure. Often in practice this will involve specifying all or at least most of the constituents.
‘Comprising’ and ‘Consisting of’
132.
The long-standing practice in the UK is that “consisting of” is generally interpreted to mean “consisting exclusively of”, [footnote 93] and thus the proportions of the specified ingredients must total 100 per cent, whilst “comprising” [footnote 94] is interpreted to mean “including” (i.e. other integers or features may be present). The phrase “consisting essentially of” is interpreted as meaning that unspecified components could be present in the claimed composition if the characteristics of the claimed composition are not materially affected by the presence of these unspecified components. This is the settled view of the EPO [footnote 95] and is followed in the UK. Thus a claim to a composition “consisting essentially of X, Y and Z” could be found to be anticipated if the prior art includes such a composition which also contains other components which do not appear to materially affect its characteristics (e.g. its activity or function). It should be noted that US practice interprets “consisting essentially of” as meaning components “that do not materially affect the basic and novel characteristic(s)”.
Numerical ranges
133.
Ranges found in patent claims are not to be treated as descriptive words or phrases, but rather as simply defining the numerical range encompassed and no further. [footnote 89] Accordingly terms like “about” and “approximately” should be objected to when used with ranges.
134.
Following the decision in Smith & Nephew Plc v Convatec Technologies Inc.[footnote 96] (as with any other feature of a claim), a numerical value or range should be construed to mean what a skilled person would have understood it to mean. [footnote 97] In this case, a claim directed towards a method of preparing an antibacterial material that required subjecting a polymer material to one or more agents “…present in a concentration between 1% and 25% of the total volume…” was held, on the facts of the case, to extend to all values ≥ 0.5% and < 25.5%. Here, the Court of Appeal concluded that whole number rounding was appropriate, dismissing the significant figures approach previously adopted by the lower court, [footnote 98] which would have resulted in the claimed range including all values ≥ 0.95% and < 25.5%. Particular importance was given to the degree of precision chosen for the claim itself (even though the description stated values having differing degrees of precision), and also what was described by the court as the mathematical quirk that arises using the significant figures approach when the bottom of the range is 1 (which results in values close to the bottom of the range being determined with much greater accuracy than those near the top). At paragraph 59 the judge held that:
…The purpose of expressing numbers to a particular degree of precision may be to convey to the reader the degree of accuracy with which he needs to make a particular measurement or carry out a calculation. In the context of the claimed method, it is to convey to the reader the range of permissible binding agent concentrations and the accuracy with which those concentrations need to be determined. There is no reason to suppose that this can vary depending upon whether the bottom of the range is 1%, 2% or 5%, or whether 10% is at the top or bottom of the range….
135.
At paragraph 37 the judge also provided some general guidance on how to approach number rounding in claims, which can be summarized as follows:
(i) The scope of any such claim must be exactly the same whether one is considering infringement or validity;
(ii) There can be no justification for using rounding or any other kind of approximation to change the disclosure of the prior art or to modify the alleged infringement;
(iii) The meaning and scope of a numerical range in a patent claim must be ascertained in light of the common general knowledge and in the context of the specification as a whole;
(iv) It may be the case that, in light of the common general knowledge and the teaching of the specification, the skilled person would understand that the patentee has chosen to express the numerals in the claim to a particular but limited degree of precision and so intends the claim to include all values which fall within the claimed range when stated with the same degree of precision;
(v) Whether that is so or not will depend upon all the circumstances including the number of decimal places or significant figures to which the numerals in the claim appear to have been expressed.
136.
The whole numbers approach to rounding was subsequently applied in Napp Pharmaceutical Holdings Limited v Dr Reddy’s Laboratories (UK) Limited [footnote 99] .
Amendments
Disclaimers
137.
A disclaimer is a form of claim limitation. It is an amendment to an already existing claim comprising the incorporation of a “negative” technical feature (see the Manual of Patent Practice at 14.126-14.127). Typically, this will entail excluding specific embodiments or areas from a general feature. In the chemical context the disclaimer is likely to relate to particular compounds, polymers etc or small groups of such substances excluded from a broader claim often in the form of a Markush formula. A disclaimer that is disclosed in the application as filed is allowed if what remains once the disclaimer has been subtracted from the claim is clearly supported i.e. it does not add matter contrary to section 76(2). An “undisclosed” disclaimer is one that is not supported by the description as filed and therefore appears to add matter contrary to section 76(2). An amendment to a claim by the introduction of an “undisclosed” disclaimer, where neither the disclaimer as such nor the subject matter excluded by it was disclosed in the application as filed, may be allowable providing certain criteria are met. These criteria were set out by the EPO Enlarged Board of Appeal in joined cases G1/03 [footnote 100] and G2/03 [footnote 100] and are recited in the Manual of Patent Practice at 14.127. The Board later confirmed in G2/10 [footnote 101] that if the criteria for allowing an undisclosed disclaimer were met the subject matter remaining in the claim after the introduction of the disclaimer would still need to be disclosed in the application as filed.
138.
Undisclosed disclaimers cannot be used to delimit a claim against non working embodiments. If a claim is directed to a large number of alternatives, some of which do not work, then either the specification will contain sufficient criteria for finding appropriate alternatives over the claimed range or there are problems relating to the sufficiency of disclosure of the invention or the level of inventive step. A disclaimer is inappropriate for dealing with either of these situations. Furthermore, the disclaimer should not remove more than is necessary either to restore novelty or disclaim excluded subject matter. A disclaimer that was relevant to the assessment of inventive step or sufficiency of disclosure would add subject matter and would not be allowed.
139.
Sudarshan v Clariant [footnote 102] concerned a pigment and its crystal forms. One of the matters under appeal concerned the lower court’s rejection of Clariant’s proposed auxiliary request which included the disclaimer “EP0361431 [the ‘517 patent] discloses the production of pigment of formula (I) as an aqueous suspension which is then filtered and washed and dried. We disclaim the said pigment as an aqueous suspension or in a form which has not been dried”, on the grounds that (i) it was not clear in its terms, and (ii) it would result in the specification disclosing additional matter. Kitchen L.J. agreed with the lower courts finding on both counts. The Court upheld the view that the disclaimer was not clear or concise with regard to the expression “in a form which has not been dried” and would therefore result in a monopoly of ambiguous and uncertain scope. In his assessment of added matter, Kitchen L.J. considered the Enlarged Board of Appeal decisions G 1/03 and G 2/03, see above, and two decisions of the English Court of Appeal, including L G Philips v Tatung, where it was held that disclaimers may be allowed to restore novelty against an “accidental anticipation” that is where “the disclosure in question must be so unrelated and remote that the person skilled in the art would never have taken it into consideration when working on the invention”. Kitchen L.J. held that not only did the disclaimer include teachings not present in the application as filed but also far from the prior art disclosure being in a remote technical field, it was in fact, the most directly relevant earlier disclosure and the starting point from which the invention of the patent in suit had been made.
Intermediate generalisation
140.
An amendment to a claim will not be allowable if by virtue of the amendment it adds subject matter. A particular example of this in the context of compositional or Markush claims is the intermediate generalisation (See the Manual of Patent Practice at 76.15.2 for a more thorough treatment of this general area). This describes the situation where a feature applying only to a narrow subclass is used instead to delimit an otherwise broader class within the scope of a claim. For example in Teva UK Ltd v Merck & Co. Inc, [footnote 103] an amended claim restricting a glaucoma treatment formulation to a particular pH range was argued to be unallowable, and this was accepted by the judge, because:
given the paucity of the disclosure about pH generally, the only disclosure that the skilled person would take out of the application as filed for combinations of dorzolamide and timolol would be gellan gum at pH 5.5 to 6.0 and HEC at pH 6. To claim a range of pH 5.5 to 6.0 for dorzolamide irrespective of viscosifier amounts to an impermissible intermediate generalisation.
Teva UK Ltd v Merck & Co, Inc [2010] FSR 17 (at paragraph 72)
141.
Pumfrey J described intermediate generalisation in Palmaz’s European Patents [footnote 104] as follows:
If the specification discloses distinct sub-classes of the overall inventive concept, then it should be possible to amend down to one or other of those sub-classes, whether or not they are presented as inventively distinct in the specification before amendment. The difficulty comes when it is sought to take features which are only disclosed in a particular context and which are not disclosed as having any inventive significance and introduce them into the claim deprived of that context. This is a process sometimes called ‘intermediate generalisation.’”
Pumfrey J in Palmaz’s European Patents [1999] RPC 47 (at page 71)
142.
The Technical Board of Appeal of the EPO has ruled that intermediate generalisations will only be allowable, and thus not add matter, if “the particular subclass … can be “directly and unambiguously derived from the application as filed”. [footnote 105]
Annex A – index of court cases and Intellectual Property Office decisions
| Case/Decision | Ref.No. | Paragraph Nos. |
|---|---|---|
| Actavis v Eli Lilly [2015] EWHC 3924 (Pat) | [footnote 36], [footnote 40], [footnote 41] | 41, 46, 72-74 |
| Actavis UK Ltd v Novartis AG [2010] EWCA Civ 82 [2010] FSR 18 | [footnote 70] | 85 |
| Adhesive Dry Mounting Co Ltd v Trapp and Co., 27 RPC 341 | [footnote 30] | 31 |
| American Cyanamid v Ethicon, [1979] RPC at page 265 | [footnote 81] | 103 |
| American Home Products Corp. v Novartis Pharmaceuticals UK Ltd [2001] RPC 8 | [footnote 84] | 110 |
| Angiotech Pharmaceuticals Inc v Conor Medsystems Inc [2007] RPC 20 (Court of Appeal) | [footnote 49] | 52,54 |
| Angiotech Pharmaceuticals Inc’s Patent (Application for Revocation by Conor Medsystems Inc) [2006] RPC 28 | [footnote 56] | 61 |
| Asahi Kasei Kogyo KK’s Application [1991] RPC 485 (House of Lords). | [footnote 3] | 4 |
| Auchincloss and another v Agricultural & Veterinary Supplies Ltd. and Others [1997] RPC 649. | [footnote 89] | 117,133 |
| Biogen Inc v Medeva plc [1997] RPC 1 | [footnote 79] | 99,108 |
| Boehringer Mannheim v Genzyme [1993] FSR 716 | [footnote 61] | 69 |
| Bristol-Myers Squibb Co v Baker Norton Pharmaceuticals Inc [1999] RPC 253 | [footnote 60] | 68 |
| British Celanese Ltd’s Application, 51 RPC 192 | [footnote 92] | 130 |
| Brugger and others v Medic-Aid Ltd [1996] RPC 635 | [footnote 59] | 66 |
| Chiron Corp v Murex Diagnostics Ltd [1996] RPC 535 (Court of Appeal) | [footnote 73] | 91 |
| Sudarshan Chemical Industries Ltd v Clariant Produkte | [footnote 102] | 139 |
| (Deutschland) GmbH [2013] EWCA Civ 919 | ||
| Conor Medsystems Inc v Angiotech Pharmaceuticals Inc [2008] RPC 28 | [footnote 55] | 61, 80, 83 |
| Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly & Co Ltd [2010] RPC 9 | [footnote 19] | 18, 19, 78-80 |
| Eli Lilly v Janssen Alzheimer Immunotherapy [2013] EWHC 1737 (Pat) | [footnote 87] | 114 |
| Evans Medical Ltd’s Patent [1998] RPC 517 (Patents Court) | [footnote 5] | 7 |
| G.E.C.’s Application, 60 RPC at page 3 | [footnote 30] | 31 |
| Genentech Inc’s Patent [1989] RPC 147 | [footnote 91] | 124 |
| General Tire & Rubber Company v Firestone Tyre & Rubber Company Limited [1972] RPC 457 at pages 485-6 | [footnote 16] | 17 |
| Generics (UK) Limited and others v H Lundbeck A/S [2009] UKHL 12 | [footnote 9] | 12, 55,107 |
| Generics (UK) Ltd v H. Lundbeck A/S [2007] R.P.C. 32 ; [2007] EWHC 1040. | [footnote 46] | 51 |
| Generics [UK] Ltd.(t/a Mylan) v Yeda Research and Development Co. Ltd & Anor [2013] EWCA Civ 925 | [footnote 65] | 80, 82 |
| Glass & Others v Freyssinet Ltd [2015] EWHC 2972 (IPEC) | [footnote 17] | 17, 39 |
| Glaxo Group Ltd’s Patent [2004] RPC 43 | [footnote 67] | 81, 88 |
| GlaxoSmithKline UK Ltd v Wyeth Holdings LLC [2016] EWHC 1045 (Ch) | [footnote 45] | 50, 51 |
| Hollister Inc’s Application [1983] RPC 10 | [footnote 77] | 97 |
| Human Genome Services v Eli Lilly [2011] UKSC 51, [2012] RPC 6 | [footnote 39] | 46 |
| Idenix Pharmaceuticals Inc. v Gilead Sciences Inc. [2014] EWHC 3916 (Pat) | [footnote 63] | 76,113 |
| I G Farbenindustrie AG’s Patent, 47 RPC 289 | [footnote 31] | 31, 78 |
| Johns-Manville Corporations Patent, [1967] RPC 479 at page 494 | [footnote 52] | 57-59 |
| Kirin-Amgen Inc. and others v Hoechst Marion Roussel Ltd and others [2005] RPC 9 (House of Lords) | [footnote 24] | 24,104,124,128 |
| Kirin-Amgen Inc. and others v. Transkaryotic Therapies Inc and others [2003] RPC 3 (Court of Appeal) | [footnote 25] | 24 |
| Medimmune Limited v Novartis Pharmaceuticals UK Limited, Medical Research Council, [2012] EWCA 1234 (Pat) | [footnote 58] | 62, 64, 73 |
| Mentor Corporation v Hollister Inc [1993] RPC 7 | [footnote 78] | 74 |
| Merrell Dow Pharmaceuticals Inc v H N Norton & Co Ltd [1996] RPC 76 | [footnote 22] | 22 |
| Napp Pharmaceutical Holdings Ltd. v Dr Reddy’s Laboratories (UK) Ltd., Sandoz Ltd. [2016] EWHC 1517 (Pat) | [footnote 99] | 136 |
| Norton Healthcare Ltd v Beecham Group Plc (BL C/62/95) | [footnote 13] | 16 |
| Novartis AG, Cibavision AG v Johnson & Johnson Medical Limited [2009] EWHC 1671 (Pat) | [footnote 86] | 112 |
| Novartis AG v Generics (UK) Limited (t/a Mylan) [2012] EWCA Civ 1623 | [footnote 50] | 55, 63, 64, 65 |
| Omnipharm Limited v Merial [2011] EWHC 3393 (Pat) | [footnote 53] | 59, 60 |
| Palmaz’s European Patents [1999] RPC 47 at 71 | [footnote 104] | 141 |
| Pfizer’s Ltd’s Patent [2001] F.S.R. 16 | [footnote 44] | 49, 68 |
| Pharmacia v Merck [2002] RPC 775 CA | [footnote 62] | 70,109 |
| Pozzoli SPA v BDMO SA [2007] EWCA Civ 588 (Court of Appeal) | [footnote 43] | 45, 48, 71, 79 |
| Prendergast’s Applications [2000] RPC 446 | [footnote 57] | 61,115 |
| Ranbaxy v Warner Lambert [2005] EWHC 2142 (Pat); [2006] FSR 14 | [footnote 14] | 17, 28, 39 |
| Raychem Corp’s Patents [1998] RPC 31 | [footnote 47] | 52 ,85,127 |
| Richardson-Vicks Inc.’s Patent [1995] RPC 568 at 581 | [footnote 68] | 81, 88 |
| Sabaf SpA v MFI Furniture Centres Ltd [2005] RPC 10 (House of Lords) | [footnote 71] | 86 |
| Saint-Gobain PAM SA v Fusion Provida Ltd and Electrosteel Castings Ltd [2005] EWCA Civ 177, [2005] IP & T 880 | [footnote 54] | 60, 67 |
| Sandvik Intellectual Property AB v Kennametal UK Ltd [2011] EWHC 3311 (Pat) | [footnote 82] | 105 |
| Smith & Nephew Plc v ConvaTec Technologies Inc. & Anor. [2013] EWHC 3955 (Pat) | [footnote 98] | 134 |
| Smith & Nephew Plc v ConvaTec Technologies Inc. & Anor. [2015] EWCA Civ 607 | [footnote 96] | 134 |
| SmithKline Beecham plc’s (Paroxetine methanesulfonate) Patent [2006] RPC 10 (House of Lords) | [footnote 2] | 4, 7, 9,12, 39 |
| Synthon BV v Smithkline Beecham plc [2005] UKHL 59 | [footnote 2] | 4 |
| Synthon B.V. v Teva Pharmaceutical Industries Limited [2015] EWHC 1395 (Pat) | [footnote 11] | 14 |
| Tate & Lyle Technology v Roquette Frères [2010] FSR 1 | [footnote 34] | 33,125 |
| Teva v AstraZeneca (asthma) [2014] EWHC 2873 (Pat, (2015) | [footnote 48] | 52, 53 |
| Teva UK Ltd v Merck & Co, Inc [2010] FSR 17 | [footnote 103] | 140 |
| Toyama Chemical Co. Ltd’s Application [1990] RPC 555. | [footnote 6] | 8 |
| Union Carbide Corp. v BP Chemicals Ltd [1998] RPC 1 | [footnote 29] | 29 |
| Windsurfing International Inc v Tabur Marine (Great Britain) Ltd [1985] RPC 59 (Court of Appeal) | [footnote 42] | 45, 48, 71, 79 |
Annex B – index of EPO Enlarged and Technical Board of Appeal decisions
available at: https://www.epo.org/law-practice/case-law-appeals/advanced-search.html unless not reported)
| Case/Decision | Ref.No. | Paragraph Nos. | |
|---|---|---|---|
| G 0001/03 | Disclaimer/PPG OJEPO 2004, 413 | [footnote 100] | 137,139 |
| G 0002/03 | Disclaimer/Genetic Systems [2004] EPOR 33 | [footnote 100] | 137,139 |
| G 0002/88 | MOBIL OIL/Friction Reducing Additive OJEPO 4/90 | [footnote 32] | 32, 33 |
| G 0006/88 | Bayer OJEPO 4/90 | [footnote 33] | 32 |
| G 0002/10 | Disclaimer/SCRIPPS OJEPO 2012 376 | [footnote 101] | 137 |
| T 0012/81 | BAYER/Diastereoisomers OJEPO 8/1982, 296 303 | [footnote 23] | 23, 28, 39 |
| T 0150/82 | International Flavours & Fragrances Inc OJEPO 1984, 309 | [footnote 26] | 24 |
| T 0181/82 | CIBA GEIGY/Spiro Compounds OJEPO 1984, 401 | [footnote 69] | 18, 84 |
| T 0198/84 | HOECHST/Thiochloroformates OJEPO 1985, 209 | [footnote 28] | 28 |
| T 0035/87 | BASF/Hydroxy-pyrazoles OJEPO 1988, 134; [1988] E.P.O.R. 260 | [footnote 76] | 95 |
| T 0081/87 | Collaborative/Preprorennin OJEPO 1990, 250 | [footnote 4] | 5 |
| T 0296/87 | HOECHST/Enantiomers OJEPO 1990, 195 | [footnote 20] | 18 |
| T 0340/89 | GENERAL FOODS/caffeine [1992] EPOR 199 | [footnote 95] | 132 |
| T 0589/89 | NATIONAL RESEARCH/Polyurethane compositions [1994] EPOR 17 | [footnote 94] | 132 |
| T 0711/90 | THE POST OFFICE (not reported) 93 132 T 0418/91 Hair Conditioner/UNILEVER | [footnote 85] | 112 |
| T 0522/91 | AMOCO (not reported) | [footnote 95] | 132 |
| T 0759/91 | AMOCO (not reported) | [footnote 95] | 132 |
| T 0939/92 | AGREVO/Triazoles 6 OJEPO 309 | [footnote 37] | 43, 44, 76, 78, 80 |
| T 352/93 | Pyrazolinverbindungen/CIBA-GEIGY (not reported) | [footnote 21] | 21 |
| T 0020/94 | Amorphous TPM/ENICHEM (not reported) | [footnote 27] | 19 |
| T 401/94 | (not reported) | [footnote 35] | 39 |
| T 0990/96 | Erythro-compounds/NOVARTIS | [footnote 10] | 13,14,15 |
| T 0812/00 | SEARLE/Cox-2 Inhibitors | [footnote 105] | 142 |
| T 0133/01 | WYETH/Dopamine agonists | [footnote 64] | 78 |
| T 0803/01 | NOVARTIS | [footnote 12] | 15 |
| T 0870/04 | Max-Planck/BDP1 phosphatase (not reported) | [footnote 74] | 92 |
| T 1329/04 | Johns Hopkins University School of Medicine/Growth Differentiation Factor [2006] EPOR 8 | [footnote 38] | 43, 44, 81 |
| T1743/06 | INEOS/Amorphous Silica | [footnote 80] | 99 |
| T 1914/11 | MacNeil AB/Liquid formulation of nicotine | [footnote 83] | 106 |
| T 2397/11 | Erlotinib hydrochloride polymorph/HOFFMANN-LA ROCHE ENANTIOMER | [footnote 8] | 11 |
| T 1555/12 | Aripiprazole polymorph/OTSUKA | [footnote 7] | 11 |
| V 0008/94 | Howard Florey Institute’s Application/Relaxin OJEPO 1995, 388 | [footnote 1] | 3 |
Footnotes
-
V 0008/94 Howard Florey Institute’s Application / Relaxin OJEPO 1995, 388 ↩ ↩2
-
SmithKline Beecham plc’s (Paroxetine methanesulfonate) Patent [2006] RPC 10 (House of Lords); Synthon BV v Smithkline Beecham plc [2005] UKHL 59 ↩ ↩2 ↩3 ↩4 ↩5 ↩6
-
Asahi Kasei Kogyo KK’s Application [1991] RPC 485 (House of Lords) ↩ ↩2
-
Evans Medical Ltd’s Patent [1998] RPC 517 (Patents Court) ↩ ↩2
-
T 2397/11 Erlotinib hydrochloride polymorph/HOFFMANN-LA ROCHE ENANTIOMER ↩ ↩2
-
Generics (UK) Limited and others v H Lundbeck A/S [2009] UKHL 12 ↩ ↩2 ↩3 ↩4 ↩5
-
Synthon B.V. v Teva Pharmaceutical Industries Limited [2015] EWHC 1395 (Pat) ↩ ↩2
-
Ranbaxy UK Limited, Arrow Generics Limited v Warner-Lambert Company [2005] EWHC 2142 (Pat); [2006] FSR 14 ↩ ↩2 ↩3
-
That is the common general knowledge at the time of the disclosure. See the Manual at 2.08 and Teva UK Ltd & Anor v Astrazeneca AB (Rev 1) [2014] EWHC 2873 (Pat) at paragraphs 75 and 76. ↩
-
General Tire & Rubber Company v Firestone Tyre & Rubber Company Limited [1972] RPC 457 at pages 485-6 ↩ ↩2
-
Glass & Others v Freyssinet Ltd [2015] EWHC 2972 (IPEC). ↩ ↩2 ↩3
-
Markush claims, named after the first patentee to successfully use claims of this form, Eugene Markush (see e.g. US1506316), are a means of claiming functionally equivalent entities such as a group of components of a composition from which one or more may be selected, or a series of compounds, notionally with the same use. They are most frequently encountered as Markush structures - a pictorial representation of a (usually) large number of compounds in one structure. This is achieved through the use of “R groups” in the structure, which represent a range of possible atoms or substituents in the molecule attached in the position indicated by the particular R group. These structures may represent many thousands of compounds. ↩
-
Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly & Co Ltd [2010] RPC 9 ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7
-
T 352/93 Pyrazolinverbindungen/CIBA-GEIGY (not reported) ↩ ↩2
-
Merrell Dow Pharmaceuticals Inc v H N Norton & Co Ltd [1996] RPC 76 ↩ ↩2
-
Kirin-Amgen Inc. and others v Hoechst Marion Roussel Ltd and others [2005] RPC 9 (House of Lords) ↩ ↩2 ↩3
-
Kirin-Amgen Inc. and others v. Transkaryotic Therapies Inc and others [2003] RPC 3 (Court of Appeal) ↩ ↩2
-
T 150/82 International Flavours & Fragrances Inc [1984] OJEPO 309 ↩ ↩2
-
Adhesive Dry Mounting Co Ltd v Trapp and Co., 27 RPC 341; G.E.C.’s Application, 60 RPC at page 3 ↩ ↩2 ↩3
-
Tate & Lyle Technology v Roquette Frères [2010] FSR 1 ↩ ↩2 ↩3
-
T 1329/04 Johns Hopkins University School of Medicine/Growth Differentiation Factor [2006] EPOR 8 ↩ ↩2 ↩3
-
Human Genome Services v Eli Lilly [2011] UKSC 51, [2012] RPC 6 ↩ ↩2 ↩3 ↩4
-
Actavis v Eli Lilly [2015] EWHC 3924 (Pat) at paragraph 178 ↩ ↩2
-
Actavis v Eli Lilly [2015] EWHC 3924 (Pat) at paragraph 177 ↩ ↩2
-
Windsurfing International Inc v Tabur Marine (Great Britain) Ltd [1985] RPC 59 (Court of Appeal) ↩ ↩2
-
Pozzoli SPA v BDMO SA [2007] EWCA Civ 588 (Court of Appeal) ↩ ↩2 ↩3
-
GlaxoSmithKline UK Ltd v Wyeth Holdings LLC [2016] EWHC 1045 (Ch) ↩ ↩2 ↩3
-
Generics (UK) Ltd v H. Lundbeck A/S [2007] R.P.C. 32 ; [2007] EWHC 1040. ↩ ↩2
-
Angiotech Pharmaceuticals Inc v Conor Medsystems Inc [2007] RPC 20 (Court of Appeal) ↩ ↩2 ↩3
-
Novartis AG v Generics (UK) Limited (t/a Mylan) [2012] EWCA Civ 1623 ↩ ↩2 ↩3 ↩4
-
T 0777/08 (Atorvastatin polymorphs/WARNER-LAMBERT) ↩
-
Johns-Manville Corporation’s Patent, [1967] RPC 479 at page 494 ↩ ↩2 ↩3 ↩4
-
Saint-Gobain PAM SA v Fusion Provida Ltd and Electrosteel Castings Ltd [2005] EWCA Civ 177, [2005] IP & T 880 ↩ ↩2 ↩3
-
Conor Medsystems Inc v Angiotech Pharmaceuticals Inc [2008] RPC 28 ↩ ↩2 ↩3 ↩4 ↩5
-
Angiotech Pharmaceuticals Inc’s Patent (Application for Revocation by Conor Medsystems Inc) [2006] RPC 28 ↩ ↩2
-
MedImmune Ltd v Novartis Pharmaceuticals UK Ltd [2012] EWCA Civ 1234 ↩ ↩2 ↩3 ↩4
-
Bristol-Myers Squibb Co v Baker Norton Pharmaceuticals Inc [1999] RPC 253 ↩ ↩2
-
Idenix Pharmaceuticals Inc. v Gilead Sciences Inc. [2014] EWHC 3916 (Pat) ↩ ↩2 ↩3
-
Generics [UK] Ltd.(t/a Mylan) v Yeda Research and Development Co. Ltd & Anor [2013] EWCA Civ 925. ↩ ↩2 ↩3 ↩4
-
It should be noted that the language used in this summary reflects the fact that the summary was formulated in the context of the EPO’s problem-solution approach. It remains the practice of the Office to utilise the Windsurfing/Pozzoli approach43 (See Lalvani et al’s patent BLO/220/13) and indeed this was the test used by the lower court in this case (see Generics [UK] LTD (t/a Mylan) v Yeda Research and Development co. Ltd & Anor [2012] EWHC 1848 (Pat)) ↩
-
Richardson-Vicks Inc.’s Patent [1995] RPC 568 at 581 ↩ ↩2 ↩3
-
Actavis UK Ltd v Novartis AG [2010] EWCA Civ 82, [2010] FSR 18 ↩ ↩2
-
Sabaf SpA v MFI Furniture Centres Ltd [2005] RPC 10 (House of Lords) ↩ ↩2
-
Available at http://www.epo.org/law-practice/legal-texts/guidelines.html ↩
-
Chiron Corp v Murex Diagnostics Ltd [1996] RPC 535 (Court of Appeal) ↩ ↩2
-
see Examination Guidelines for Patent Applications Relating to Biotechnological Inventions paragraph 60 ↩
-
T 35/87 BASF/Hydroxy-pyrazoles OJ EPO 1988, 134; [1988] E.P.O.R. 260 ↩ ↩2
-
American Cyanamid v Ethicon, [1979] RPC 215 at page 265 ↩ ↩2
-
Sandvik Intellectual Property AB v Kennametal UK Ltd [2011] EWHC 3311 (Pat) ↩ ↩2
-
American Home Products Corporation & Anor. v Novartis Pharmaceuticals UK Ltd & Anor. [2000] EWCA Civ 231, [2001] RPC 8. ↩ ↩2
-
Novartis AG, Cibavision AG v Johnson & Johnson Medical Limited [2009] EWHC 1671 (Pat) ↩ ↩2
-
Eli Lilly v Janssen Alzheimer Immunotherapy [2013] EWHC 1737 (Pat) ↩ ↩2
-
See Examination Guidelines for Patent Applications relating to Medical Inventions paragraphs 206-209. ↩
-
Auchincloss and another v Agricultural & Veterinary Supplies Ltd. and Others [1997] RPC 649. ↩ ↩2 ↩3
-
University of Rochester v G.D. Searle & Co., Inc 358 F. 3d 916 (Federal circuit 2004) ↩
-
This is consistent with EPO practice e.g. T 711/90, unreported ↩
-
This is consistent with EPO practice e.g. T 589/89 NATIONAL RESEARCH/Polyurethane compositions [1994] (EPOR 17) ↩ ↩2
-
T 340/89 GENERAL FOODS/caffeine ([1992] EPOR 199), T 522/91 and T759/91(each unreported)) ↩ ↩2 ↩3 ↩4
-
Smith & Nephew Plc v Convatec Technologies Inc. [2015] EWCA Civ 607 ↩ ↩2
-
In accordance with the decision in Kirin-Amgen Inc. and others v Hoechst Marion Roussel Ltd and others [2005] RPC 9 (House of Lords) ↩
-
Smith & Nephew Plc v Convatec Technologies Inc & Anor [2013] EWHC 3955 (Pat) ↩ ↩2
-
Napp Pharmaceutical Holdings Limited v Dr Reddy’s Laboratories (UK) Limited, Sandoz Limited [2016] EWHC 1517 (Pat) ↩ ↩2
-
G1/03 Disclaimer/PPG and G2/03 Disclaimer/Genetic Systems [2004] 8-9 OJEPO 413 and [2004] EPOR 33 ↩ ↩2 ↩3 ↩4
-
Sudarshan Chemical Industries Ltd v Clariant Produkte (Deutschland) GmbH [2013] EWCA Civ 919 ↩ ↩2
