UK Influenza Network Laboratories on interpretation of influenza-positive results: 2021 to 2022 season
Published 17 November 2021
Background
The introduction of the UK childhood influenza vaccination programme began in autumn 2013. This 2021 to 2022 season, a single dose of live attenuated influenza vaccine (LAIV), Fluenz® Tetra, should be offered to all children who are aged 2 to 15 on 31 August 2021, and eligible risk-group adolescents aged 16 to less than 18 (1, 2). Children aged 2 years to less than 9 years old in a clinical risk category and receiving influenza immunisation for the first time should receive repeat LAIV after a 4-week interval. If LAIV is contraindicated, or not acceptable, an intramuscular cell-based quadrivalent influenza vaccine (QIVc) can be offered instead.
Fluenz® Tetra contains live-attenuated influenza viruses of the following 4 strains in 2021 to 2022 (3):
- A/Victoria/2570/2019 (H1N1)pdm09-like strain
- A/Cambodia/e0826360/2020 (H3N2)-like strain
- B/Washington/02/2019 (B/Victoria lineage)-like strain
- B/Phuket/3073/2013 (B/Yamagata lineage)-like strain
These attenuated virus strains undergo viral replication in respiratory tissues (for example, nasal passages) which are of lower temperature than internal body temperature. Since the nasal passages are infected with live influenza virus vaccine strains after LAIV administration, sampling the nasal passages within a few days after LAIV vaccination can yield positive influenza testing results.
Studies in children and adults from 2008 and 2011 detected shedding with viral culture post LAIV (4, 5). These indicated that maximal shedding typically occurs within 2 days of vaccination. It may be possible to detect LAIV vaccine strains up to 11 days post vaccination – detection of shedding >11 days post vaccination was uncommon and occurred almost exclusively in children 6 to 23 months of age (an age group for which LAIV is not licensed). Later studies have used PCR to detect viral shedding which is more sensitive than viral culture. A study of shedding after two 2015 to 2016 LAIV formulations, and one 2016 to 2017 formulation, showed shedding of quantifiable strains from day 2 to day 7 post-vaccine administration (6). Shedding decreased after second dose of LAIV.
Detection of LAIV virus shedding post vaccination in childhood vaccine campaign
The LAIV vaccination campaign targeting children normally begins in early autumn every year. Following the implementation of the campaign, occasionally children who are within 14 days of their vaccine may inadvertently or unexpectedly have a respiratory virus swab taken, either as part of intercurrent illness investigation in a sentinel primary care setting, or on review/admission to hospital. As a result, clinical diagnostic laboratories may receive samples which on routine testing for respiratory viruses have an unusual pattern of reactivity, where more than one influenza virus is detected. A positive result in a child who recently received intranasal administration of LAIV may therefore indicate detection of vaccine virus.
Following the seasonal delivery of the LAIV programme, the possibility of obtaining misleading (falsely positive for wild-type influenza infection) positive results should be considered in children who have received the LAIV within the preceding 2 weeks, and subsequently have upper respiratory tract samples tested for influenza virus (7, 8). Clinical studies undertaken by the UK Health Security Agency (UKHSA) on virus shedding in children following LAIV in 2 winter seasons (10) (see Appendix 1) demonstrate the following principles:
- Influenza B virus is the most likely vaccine virus to be detected.
- Younger children receiving their first dose are more likely to shed detectable vaccine virus.
- There is variation year to year in the way in which live attenuated vaccine strains may replicate, so that the patterns of reactivity cannot always be predicted.
- The timing of detection of vaccine viruses is typically maximum within the first week after vaccination.
The detection of influenza co-infection in a sample from an individual <16 years taken in winter months should raise the suspicion that this is a sample taken from a recent recipient of LAIV vaccine, and a follow-up enquiry undertaken.
The detection of LAIV vaccine strains in influenza-positive samples can be confirmed in the UKHSA reference laboratory (Respiratory Virus Unit (RVU), Colindale) by whole genome sequencing. This demonstrates that the internal gene segments of the detected virus are derived from the master Ann Arbor donor vaccine strains, and are not similar to circulating strains, whereas the sequence analysis of the surface protein genes (haemagglutinin and neuraminidase) will not necessarily be distinguishable from circulating strains.
Appendix 2 and Appendix 3 show the distribution of samples received by the RVU from vaccinated children in the UK, in which shedding of LAIV was confirmed. Appendix 2 shows a graph of samples from 2013 to 2016, demonstrating viral shedding in days 2 to 4 and 6 to 9, post vaccination. Appendix 3 shows a graph of samples from 2020, demonstrating viral shedding in days 1, 4 to 7, 10 and 13 post vaccination.
Interpretation of the results of molecular testing of samples
The following 2 tables provide guidelines on the interpretation of molecular testing results for influenza detection. It is recognised that vaccination and contact history will rarely be available at the time of reporting, and laboratories should take this into account when devising result report comments.
Table 1. Patients who have no history of vaccination with LAIV and no history of contact with a vaccine recipient in the preceding 2 weeks
| Typing PCR result | Subtyping (H1)pdm09 PCR result | Subtyping (H3) PCR result | Interpretation |
|---|---|---|---|
| A | Positive | Negative | A(H1)pdm09 wild type strain detected |
| A | Negative | Positive | A(H3) wild type strain detected |
| B | Negative | Negative | Influenza B wild type strain detected |
| A and B | Positive | Negative | A(H1)pdm09 and B wild type strains detected Dual infection 1 |
| A and B | Negative | Positive | A(H3) and B wild type strains detected Dual infection1 |
| A and B | Positive | Positive | A(H1)pdm09, A(H3) and B wild type strains detected Co-infection2 |
| Negative | Negative | Negative | Negative for influenza |
1 Repeat testing is recommended for samples indicating dual infections or equivocal results. Detection of more than one influenza type and/or subtype could reflect serial infections with prolonged detection of the first infecting virus.
2 Repeat testing and investigate patient’s vaccination history and/or contact with vaccine recipient. Very unlikely to be wild-type; laboratory confirmation of false positive(s) due to LAIV contamination can only be performed by sequence analysis.
Table 2. Patients who have received LAIV within the preceding 2 weeks or have been in contact with an individual who has a history of vaccination with LAIV1 in the preceding 2 weeks
| Typing PCR result | Subtyping (H1)pdm09 PCR result | Subtyping (H3) PCR result | Interpretation |
|---|---|---|---|
| A | Positive | Negative | A(H1)pdm09 detected Possible LAIV detection* |
| A | Negative | Positive | A(H3) detected Possible LAIV detection* |
| B | Negative | Negative | Influenza B detected Possible LAIV detection* |
| A and B | Positive | Negative | A(H1)pdm09 and B detected Possible LAIV detection* |
| A and B | Negative | Positive | A(H3) and B detected Possible LAIV detection* |
| A and B | Positive | Positive | A(H1)pdm09, A(H3) and B detected Possible LAIV detection* |
| Negative | Negative | Negative | Negative for influenza |
1 There is a theoretical potential for transmission of live attenuated influenza virus in Fluenz® Tetra to immunocompromised contacts for one to two weeks following vaccination. In the US, where there has been extensive use of the LAIV, there have been no reported instances of illness or infections from the vaccine virus among immunocompromised patients inadvertently exposed. In theory, healthcare workers may have low-level exposure to LAIV viruses during administration of the vaccine and/or from recently vaccinated patients. The vaccine viruses are cold-adapted and attenuated and are unlikely to cause symptomatic influenza. In the US, where there has been extensive use of LAIV, no transmission of vaccine virus in healthcare settings has ever been reported and there have been no reported instances of illness or infections from the vaccine virus among healthcare professionals inadvertently exposed (9).
*Laboratory confirmation of suspected LAIV detection can only be performed by sequence analysis.
Clinicians may decide to continue (or commence) antiviral therapy in a patient who has an influenza-like illness in the context of recent LAIV administration, if the patient is otherwise a candidate for treatment of influenza (as per the relevant NICE Technology Appraisal – see also UKHSA antiviral guidelines). The results of reference tests to determine the presence of LAIV versus circulating seasonal influenza viruses are unlikely to be available in a clinically useful time frame. Infection with other respiratory viruses is a possibility – rhinovirus and respiratory syncytial virus (RSV) may be prevalent when LAIV is typically given. Administration of influenza antiviral agents within 2 weeks of vaccination may affect response to the vaccine.
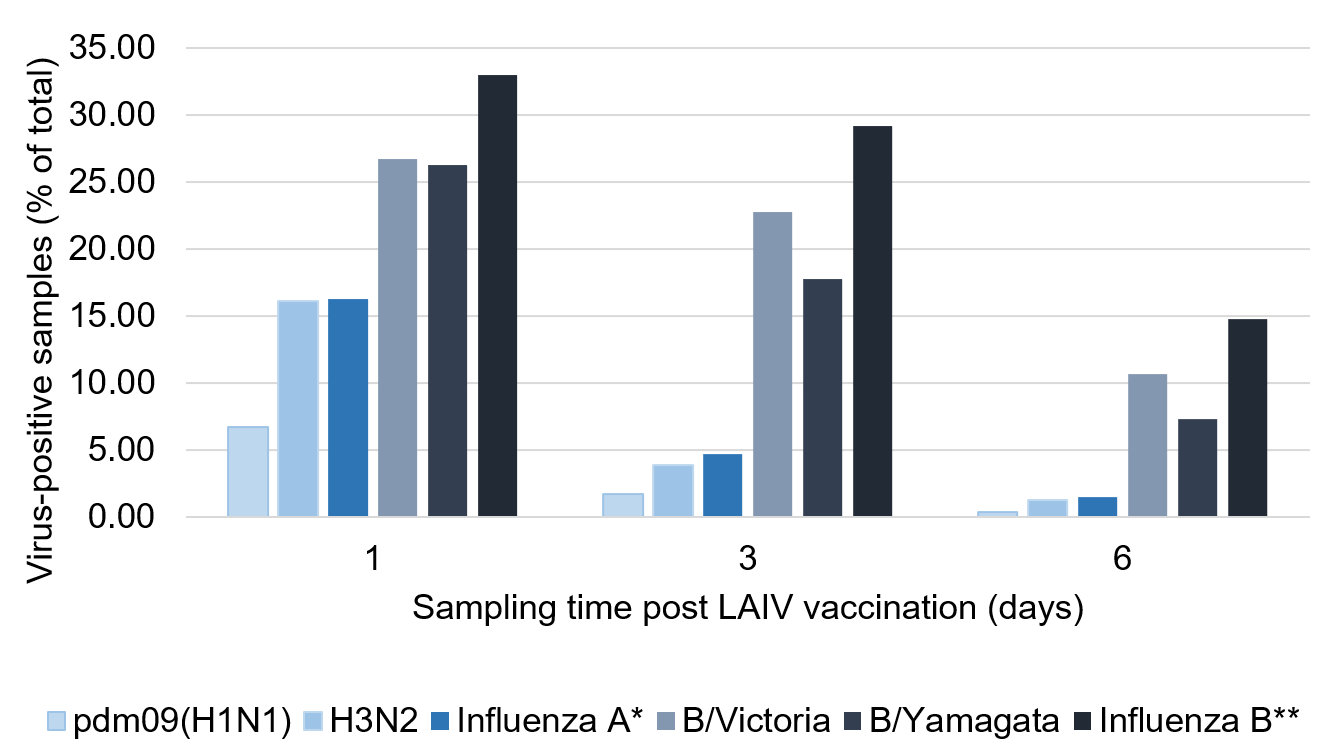
Appendix 1. Viral shedding in the 6 days post 2016 to 2017 or 2017 to 2018 LAIV vaccination (10)
*Percentage of study participants that were positive for pdm09(H1N1)-like and/or H3N2-like LAIV viruses
**Percentage of study participants that were positive for B/Victoria-like and/or B/Yamagata-like LAIV viruses
Abbreviations:
B/Vic = B/Victoria; B/Yam = B/Yamagata; H1 and H3 = haemagglutinin subtype
The figure in Appendix 1 shows the percentage of total samples analysed that were positive for each subtype on respiratory samples taken on day 1, 3 and 6 following 2016 to 2017 or 2017 to 2018 LAIV administration (groups combined). Peak shedding times were on day 1 post vaccination for influenza A viruses and between days 1 to 3 for influenza B viruses (with the caveat that it is impossible to know what proportion of positivity was actually due to input virus and therefore not necessarily shedding, especially on day 1).
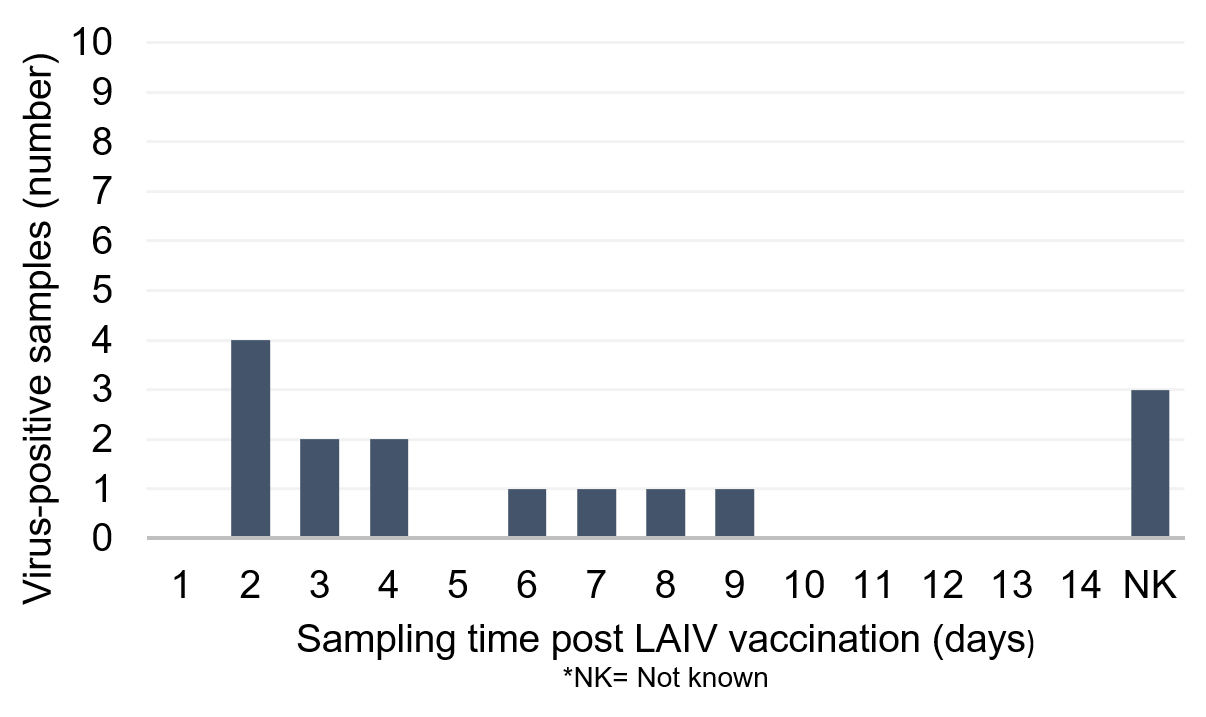
Appendix 2. Distribution of sentinel and non-sentinel samples received from vaccinated children in the UK 2013 to 2016 in which shedding of LAIV was confirmed by sequence analysis in RVU, Colindale
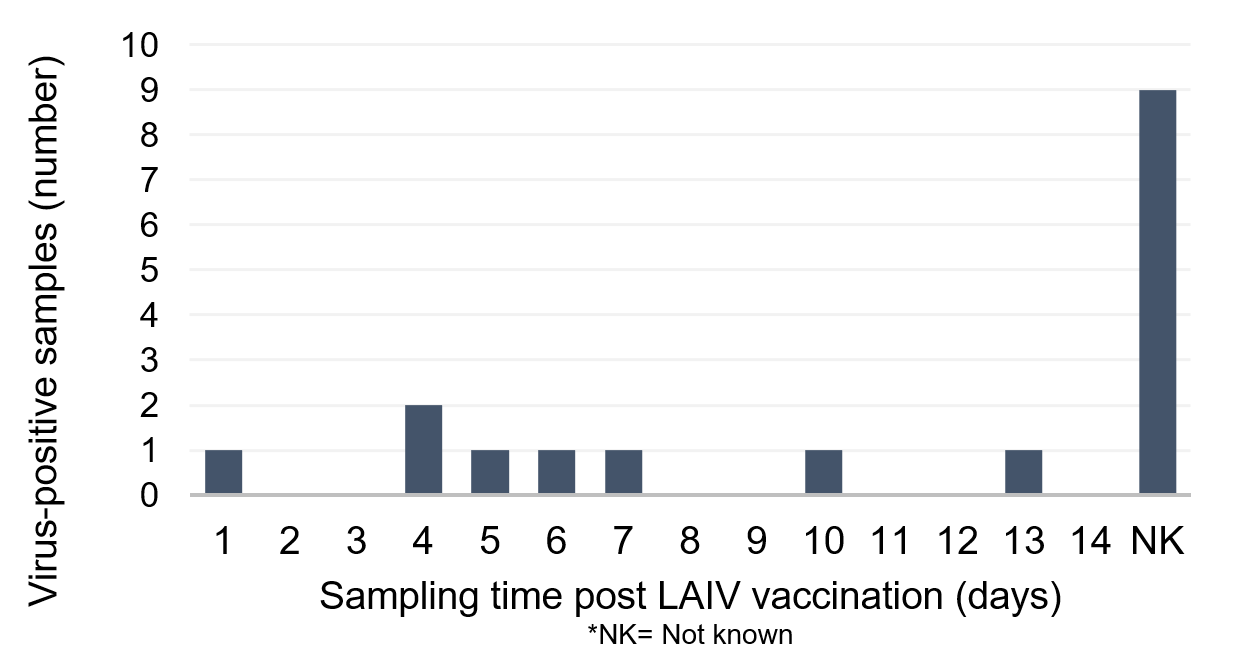
Appendix 3: distribution of sentinel and non-sentinel samples received from vaccinated children in the UK 2020, in which shedding of LAIV was confirmed by sequence analysis in RVU, Colindale
References
1. Department of Health and Social Care (DHSC). National flu immunisation programme 2021 to 2022 letter. DHSC; 2021.
2. UK Health Security Agency (UKHSA). ‘Live attenuated influenza vaccine nasal spray suspension (LAIV) Patient Group Direction (PGD) v10.00’. UKHSA; 2021.
3. Electronic Medicines Compendium. Fluenz Tetra nasal spray suspension Influenza vaccine (live attenuated, nasal) – Summary of Product Characteristics (SmPC). 2021.
4. Block S, Yogev R, Hayden F, Ambrose C, Zeng W, Walker R. ‘Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5-49 years of age’. Vaccine 2008; volume 26, issue 38, pages 4,940-6.
5. Mallory R, Yi T, Ambrose C. ‘Shedding of Ann Arbor strain live attenuated influenza vaccine virus in children 6-59 months of age’. Vaccine 2011; volume 29, issue 26, pages 4,322-7.
6. Mallory R, Nyborg A, Kalyani R, Yuan Y, Block S, Dubovsky F. ‘A study to evaluate the immunogenicity and shedding of live attenuated influenza vaccine strains in children 24-<48 months of age’. Vaccine 2020; volume 38, issue 5, pages 1,001-1,008.
7. Jin H, lu B, Zhou H, Ma C, Jackie Z, Yang C, and others. ‘Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (flumist) derived from cold-adapted a/ann arbor/6/60’. Virology 2003: volume 306, issue 1, pages 18-24.
8. Chan W, Zhou H, Kemble G, Jin H. ‘The cold adapted and temperature sensitive influenza A/Ann Arbor/6/60 virus, the master donor virus for live attenuated influenza vaccines, has multiple defects in replication at the restrictive temperature’. Virology 2008: volume 380, pages 304-11.
9. UKHSA. Green book chapter 19 influenza.
10. Jackson D, Pitcher M, Hudson C, Andrews N, Southern J, Ellis J and others. ‘Viral Shedding in Recipients of Live Attenuated Influenza Vaccine in the 2016-2017 and 2017-2018 Influenza Seasons in the United Kingdom’. Clinical Infectious Diseases 2020. Volume 70, issue 12, pages 2,505-2,513.
