HPR volume 9 issue 33: news (18 September)
Updated 29 December 2015
1. ACMP malaria prevention guidelines updated
The Advisory Committee on Malaria Prevention (ACMP), an expert advisory committee of Public Health England (PHE), has published its annual revision of the ‘Guidelines for malaria prevention in travellers from the UK’ for 2015 [1].
These guidelines are a practical guide for medical professionals and other travel medicine advisors based in the UK who advise travellers, and may also be of use to travellers who wish to read about the options themselves.
The key changes included in the 2015 revision include:
- updated guidance on the use of insect repellent and sun protection
- clarification on the use of hydroxychloroquine
- updated guidance on the use of anticoagulants with antimalarials
- updated guidance on the use of doxycycline in epilepsy
- changes to the country recommendations for Vietnam and Malaysian Borneo, and clarifications on the recommendations for India
- additional notes added at the beginning of the country recommendations table including information on vulnerable travellers, and new malaria maps for India and South Africa
- clarification of advice for travellers moving through areas where different antimalarials are recommended
- details about the ACMP have been added including: membership, terms of reference and methodology used to make recommendations (these details are now also available on the PHE website [2]).
It is important that recommendations for antimalarials should be appropriate for the destination and tailored to the individual, taking into account possible risks and benefits to the traveller. As part of an individual stringent risk assessment, it is essential that a full clinical history is obtained, detailing current medication, significant health problems and any known drug allergies. A suggested risk assessment template is included with the guidelines.
While the focus of these guidelines is on malaria prevention, it should be emphasised that malaria prevention is only one aspect of pre-travel advice. A comprehensive risk assessment-based package of travel health advice should be provided to travellers 6-8 weeks (ideally) before they travel. Travel health advice is available from the National Travel Health Network and Centre (NaTHNaC) website at: http://travelhealthpro.org.uk/.
1.1 References
- PHE (17 September 2015). Malaria prevention guidelines for travellers from the UK: 2015.
- ACMP webpage.
2. Mandatory HCAI reports quarterly trends: April to June 2015
PHE’s latest quarterly epidemiological commentary on trends in reports of Staphylococcus Aureus (MRSA and MSSA) and Escherichia Coli bacteraemia, and of Clostridium Difficile infections, mandatorily reported by NHS acute Trusts in England up to April-June 2015, has been published on the GOV.UK website [1].
The report, including tabular and graphical information, provides data for the April-June 2015 quarter (updating the previous report published in June 2015). Some key facts are listed below.
2.1 MRSA bacteraemia
There has been a 21.3% decrease (1.97 to 1.55 reports per100,000 population) in rates of total MRSA bacteraemia reports between January-March 2012 and the current quarter (April-June 2015). This is part of an overall decreasing trend beginning from April 2007. However, more recently (April-June 2014 to April-June 2015) increases in both the counts and rates of total MRSA bacteraemia have been reported (from 181 to 210 and from 1.34 to 1.55 per 100,000 population, respectively). This has been observed for Trust-assigned (from 73 to 86 and from 0.85 to 1.00 per 100,000 bed-days), CCG-assigned (from 91 to 98 and from 0.67 to 0.72 per 100,000 population) and Third Party-assigned cases (from 17 to 26 and from 0.13 to 0.19 per 100,000 population). The increases in Trust- and CCG-assigned reports and rates represent the first year-on-year inter-quarter increase since the PIR process was initiated (April 2013).
2.2 MSSA bacteraemia
The current quarter (April-June 2015) saw the highest rate of total MSSA bacteremia (18.93 reports per 100,000 population) since the reporting of MSSA bacteraemia cases was initiated in January 2011. The count of total MSSA bacteraemia has increased by 10.8% in the current quarter (April-June 2015, n=2,564) when compared to the same quarter in the previous year (April-June 2014, n=2,315). Conversely, there has been little change in the counts of Trust apportioned MSSA bacteraemia reports within the same time period, with a 0.4% decrease in counts from 682 to 679 reports.
2.3 E Coli bacteraemia
A 2.9% increase (from 65.62 to 67.49 reports per 100,000 population) has been observed in the rate of E. Coli bacteraemia reports when comparing the current quarter (April-June 2015) with the same quarter of the previous year (April-June 2014), with an overall increase of 16.6% (from 57.88 to 67.49 reports per 100,000 population since January-March 2012). The increase in E. Coli bacteraemia reports and rates between April-June 2014 and April-June 2015 represent the ninth consecutive increase since April-June 2013 between a quarter and the same quarter in the previous year.
2.4 C. Difficile infection (CDI)
From April-June 2014 to April-June 2015 there was a 6.2% increase in the counts and rates of total CDI reported from 3,442 to 3,654 reports and 25.42 to 26.98 reports per 100,000 population respectively. Similarly within the same period, counts and rates of the Trust-apportioned CDI reported have both increased by 9.9% (from 1,197 to 1,316 reports and 13.97 to 15.36 reports per 100,000 bed-days). This is now the fourth consecutive observed increase in counts and rates of Trust-apportioned CDI and the fifth for counts and rates of total reported CDI, when comparing to the same quarters in the previous year.
2.5 Reference
- PHE (10 September 2015). Quarterly Epidemiological Commentary: Mandatory MRSA, MSSA and E. coli bacteraemia, and C. difficile infection data (up to April-June 2015).
3. Ebola virus disease: international epidemiology summary (at 13 September 2015)
The West African Ebola Virus Disease (EVD) outbreak continues with a total of 28,256 clinically compatible cases (15,233 confirmed) reported as of 13 September 2015, 11,306 of which have died.
There were five confirmed cases reported in the past week (see figure), all in Sierra Leone, compared to one in Guinea and one in Sierra Leone in the previous week.
This is the first time Guinea has recorded an EVD-free week for over 12 months. Four of the five cases reported this week in Sierra Leone are associated with the previously reported cluster in Kambia. All four are close relatives of the initial case.
The remaining case in Sierra Leone was reported in Bombali, a district that had not reported a case for over five months. Investigation into the source of infection is ongoing. The case was symptomatic in the community for several days before being admitted to an Ebola treatment centre and further cases are expected.
A total of 1,785 contacts remain under follow up (241 in Guinea and 1,524 in Sierra Leone), rising from 1,281 in the previous week.
Liberia remains within a 90 day period of heightened vigilance following being declared EVD transmission free on 3 September 2015.
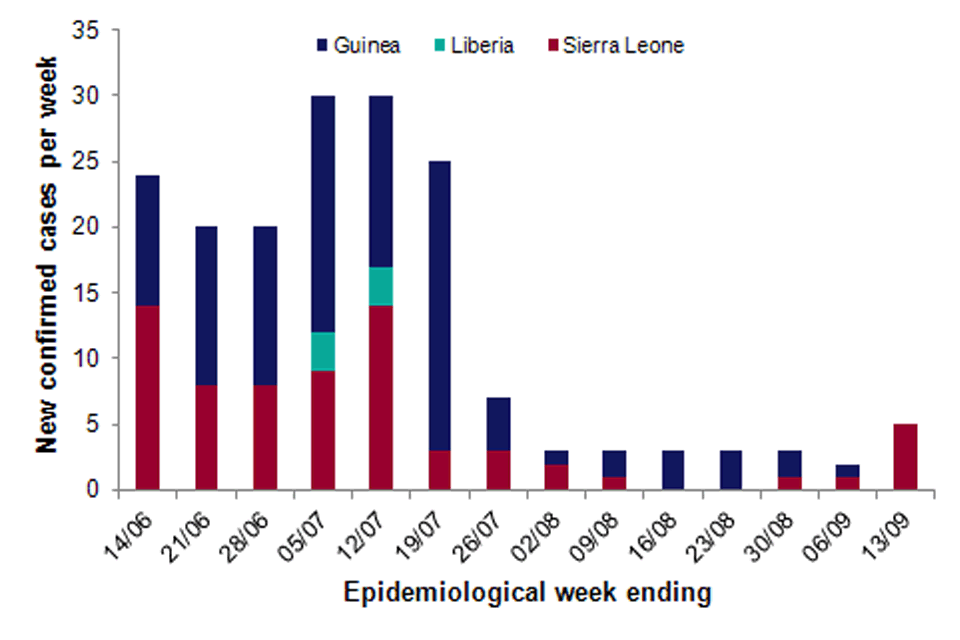
Number of new confirmed cases reported per week (14 June to 13 September 2015) in affected countries in West Africa. (Data source: WHO Ebola Situation Report 16 September 2015)
Further information on the epidemiological situation can be found in PHE’s weekly Ebola epidemiological update and from the Ebola outbreak distribution map.
4. Annual update on voluntarily reported candidaemia published
In England, Wales and Northern Ireland the overall rate of Candida species blood infections was 2.8 per 100,000 population in 2014, according to the annual report published in the infection reports section of this issue of HPR [1].
In 2014, the top three reported Candida species in blood infections were: Candida Albicans (45%), as C. Glabrata (26%) and C. Parapsilosis (10%). Reports of fungaemia caused by Candida Albicans decreased by 14% over the survey period, 2010 to 2014. The rate of candidaemia was highest in those aged 75 years and over, a pattern reflected in the top three reported candida species.
Antifungal susceptibility data for 2014 for the top three reported Candida species are also included in the report.
4.1 Reference
- PHE (September 2015). Surveillance of candidaemia in England, Wales and Northern Ireland: 2014, HPR 9(33): bacteraemia, 18 September.
