Bovine tuberculosis in Great Britain in 2021: Explanatory supplement to the annual reports
Updated 7 December 2022
Applies to England, Scotland and Wales
Preface
This Explanatory Supplement is produced to support annual reports that describe the surveillance data and epidemiology of bovine tuberculosis (TB) in Great Britain, specifically the reports titled:
- ‘Bovine tuberculosis in Great Britain-Surveillance data for 2021 and historical trends’; referred to as the ‘2021 GB TB data report’
- ‘Bovine tuberculosis in England 2021: Epidemiological analysis of the 2021 data and historical trends’’; referred to as the 2021 England TB Epi report
- Bovine tuberculosis epidemiology reports 2021 for counties in the Low Risk Area of England
The content of this explanatory supplement is derived from explanatory text provided in previous annual reports to describe data, methodology and definitions and is updated where appropriate. It is split into 4 sections:
- Background of TB in Great Britain
- Methodology for the TB reports
- Overview of TB testing in Great Britain
- Policies for TB control in Great Britain
1. Background of TB in Great Britain
1.1. Overview of TB transmission pathways
In Great Britain the main species infected with Mycobacterium bovis, the bacterium that causes bovine tuberculosis (TB), are domestic cattle and wild badgers. Figure 1.1 shows the simplified TB transmission pathways involving cattle and badger populations in terms of the spread of the disease.
Figure 1.1 Simplified potential TB transmission pathways involving cattle and badgers.

TB transmission can occur between cattle herds and between badger social groups. Direct transmission can occur between cows and badgers. Indirect transmission occurs when cattle or badgers infect the environment, and the environment infects them.
However, there is a wide variety of potential routes the bacteria can pass between the 2 main host species. These may be facilitated by the ability of M. bovis to survive in the environment for many months. Figure 1.2 shows the wide range of risk factors and pathways by which cattle can become infected with TB.
Figure 1.2 Detailed diagram showing TB potential risk pathways
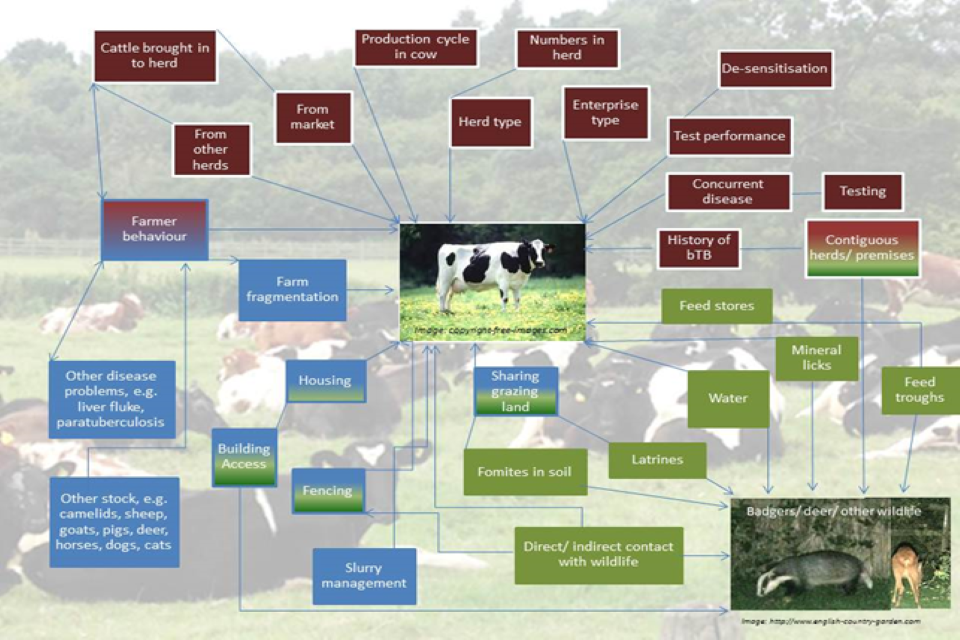
Most of the brown box variables in Figure 1.2 can be determined remotely from government datasets, such as assessing cattle movements using the Cattle Tracing System (CTS). However, some movements are not reported, such as movements within 10 miles between premises under the same ownership. The numbers of cattle in the herd are variable and herd size is affected by changing management practices during the year. These changes arise from expansion, loss to disease or maintaining a varied genetic pool. They also include ad hoc management practices that are not captured by remote systems, such as varying the composition of the different epidemiological groups. Herd and enterprise types and concurrent disease can be difficult to establish remotely and are subject to change. Test performance depends on both, test characteristics (meaning individual vs group level), but also on correct application and interpretation. History of TB and testing records are readily available (although reliability is dependent on the quality of data entry). The presence of contiguous neighbours can be assessed using land ownership data, but this can obscure differences between ownership and use of the land.
The variables in green and blue boxes depend on knowledge of farm management practices. These can often only be established through farm visits and by direct contact with the farmer. Geographic Information Systems (GIS) can help define the extent of farm fragmentation; however, it is difficult to provide certainty with data and methodology when applied to a large number of herds.
It should be noted that the risk pathways outlined in Figure 1.2 are potential risk pathways and as such, are dependent on the level of exposure to the hazard on a particular farm.
2. Methodology used for the TB reports
2.1 Data sources and processing
2.1.1 Source of data
Data on herds, animals, bovine tuberculosis incidents and tests applied to British cattle were downloaded from the APHA SAM RADAR TB reception database on 12 May 2022. This includes skin tests entered on to SAM and completed on or before the 20 May 2022. Data prior to late September 2011 derives from the old VetNet system, which was decommissioned and migrated into SAM at the end of September 2011. Information relating to culture results of all TB suspect samples exists on SAM. This is derived from the APHA’s LIMS system for samples from around the time of SAM TB going live. Prior to that, sample information came from the APHA TB Culture System (TBCS), but there was a short cross-over period when both were in use. Apparent missing results on SAM have been retrieved directly from LIMS where possible, particularly during the live launch of SAM TB.
Data are downloaded at least 3 months after the reporting year. This is to capture as many laboratory culture results for incidents commencing in the reporting year as possible. However, this date is too early to capture all events during most of these incidents. An example of this is the dates of removal of movement restrictions from which the duration of incidents is calculated. Therefore, incidents that ended during the reporting year are used to calculate the duration of incidents and the total number of reactors in an incident.
As in previous reports, the old county boundaries that were set in 1974 are used throughout.
In England, outputs are for the most part broken down by the new TB risk and surveillance areas introduced in 2013. From January 2018, part Edge, part HRA split counties were re-classified as fully Edge Area. These include Cheshire, Derbyshire, East Sussex, Oxfordshire, and Warwickshire. Herds in these counties are subject to mandatory interferon gamma blood testing in TB breakdowns with lesion and/or culture positive animals. Herds in some parts of the Edge Area are subject to 6-monthly routine surveillance testing. These are located in all or part of Cheshire, Derbyshire, East Sussex, Hampshire, Oxfordshire and Warwickshire. Others remain on annual testing supplemented with 3km radial testing around TB breakdown herds with lesion and/or culture positive animals.
Considerable effort has been made to correct as many of the data inconsistencies observed in SAM as possible. As such, we are confident we have used a dataset that is broadly correct. However, there still may be small differences in incident numbers with the National TB Statistics published every month by the Defra statistics team in York. The APHA TB Epidemiology and GB Data Reports and the National Statistics are produced and published in different ways. The APHA Reports are published the following calendar year, whereas the National Statistics data are published 3 months after data becomes available. A consequence of this prompt publication is that there is less scope to check and clean the data in the National Statistics prior to publication.
In most cases, discrepancies between the APHA reports and the National Statistics are a result of the following issues:
- Some TB incidents do have a start date in SAM that lags behind the true disclosing test date. This may result in placing it in a different period in SAM to the APHA reports.
- Inaccurate or missing TB10 end of restriction information has also been a serious issue within SAM and one addressed since the 2012 report. Revisions in SAM, policy changes and user training have reduced many errors in this respect and the situation is now greatly improved. There are still a very small number of incidents with obviously incorrect or missing TB10 dates which have been or may still be corrected. Closure of incidents involves the receipt of a BT5 form which provides evidence of cleansing and disinfection on the incident premises. This is required before a TB10 can be issued to formally close the incident. Non-receipt or delays in the receipt of the BT5 form will artificially prolong the duration of incidents, should last until the final clearing skin test. Policy introduced late in 2015 has attempted to penalise non-returners of the BT5 and the situation appears to be much improved. A similar delay can also be due to noted discrepancies within the BCMS Cattle Tracing System. If animals observed on the farm do not all match those reported in BCMS, this must be corrected prior to the lifting of restrictions. There are occasionally administrative errors where incidents do not appear on SAM, despite evidence of reactors and short-interval tests occurring in those holdings. These are true new incidents and are counted in the APHA reports, but are few.
- There are also additional incidents that are counted by National Statistics, but are removed during data cleansing and not represented in the APHA Reports. For example, administrative errors where multiple incidents are created simultaneously under the same herd can occur. Herds with culture-negative slaughterhouse cases in which no reactors are found and herds with NVL 1x IRs only are not considered new incidents in this report.
- Work is continually conducted to harmonise reporting between APHA Reports and the National TB Statistics. This aims to establish as similar an underlying dataset as possible.
2.1.2 Classification of incidents
Since January 2011, cattle herds in GB have been described by their Official Tuberculosis Free (OTF) status. This can be OTF-W (OTF withdrawn), OTF-S (OTF suspended), or if free from any restrictions, OTF. This terminology is used in the surveillance reports.
OTF-S incidents describe herds in which all test reactors failed to disclose visible lesions or positive laboratory results. Other herds without a TB incident can have their OTF status temporarily suspended under the following conditions:
- observation of suspect TB lesions in a slaughterhouse during routine post-mortem meat inspection and pending a culture result
- overdue routine tests for a herd
- when IRs only are found within 3 years of a previous OTF-W incident in the same herd
However, such herds do not contribute to OTF-S incident totals in this report. In some figures differentiation is made between OTF-S incidents with 0-1 or >1 reactor. Few are classed as ‘unclassified’, where no post mortem results are available.
For the purpose of this report, OTF-W status refers to a herd with a TB incident in which:
- the presence of tuberculosis is confirmed in at least one animal by positive laboratory confirmation of M. bovis infection
- classical lesions of tuberculosis are seen at post mortem examination in the carcass of at least one test reactor animal from the herd
TB incidents in Wales where an epidemiological assessment establishes the likelihood of infection without post-mortem evidence of infection can also be classified as OTF-W. These are technically termed OTF-W-2, but not differentiated from OTF-W within these reports.
To qualify as being “new” within the specified period, a TB herd incident must have been disclosed and restrictions imposed within that period.
2.2. Epidemiological Indicators of disease burden
2.2.1 Incidence
Incidence is the rate of new cases that occur in a population of interest over a specified period of time. Successful control of an epidemic should be reflected by a reduction in the probability that new cases will occur. This can be difficult to measure if detection of infection is dependent on proactive testing of herds at different frequencies. Different approaches can vary values slightly, so it is important to understand the characteristics of the measure being used. Comparing areas over time thus also need to use the same consistent measures. International notifications of disease status often require a measure of incidence.
The incidence rate used in the National Statistics for Great Britain is the number of new incidents per 100 herd-years at risk (HYR). HYR is the sum of the time (in days, months or years) that herds in the geographical area of interest are unrestricted. The incidence per 100 HYR therefore gives the number of new cases over 100 herd-years. This measure considers the historical testing frequency and the periods that a herd is classified as un-restricted and at risk of infection. Only herds that have a test during the reporting period contribute to the measure. This index is generally considered more accurate for comparing incidence between areas as it accounts for different intervals between tests in herds that other measures do not.
The method for calculating incidence per 100 HYR has been modified from that described by Downs et al. (2012). The numerator is the total number of new TB incidents detected in the year in the area of interest. The denominator is the sum of the time during which all herds in the same area are considered at risk of a TB incident (time at risk). A herd was considered to be at risk of a TB incident between:
- negative herd tests (herd tests clear of infection)
- a negative herd test and the disclosure of a TB incident, and
- from the end of movement restrictions (date of TB10) after a TB incident to the next herd test
The time at risk is calculated as the total time the herd was not under restriction since the most recent test or end of restrictions before or at the beginning of the year. Only periods of risk that end in the time period for which the rate is being estimated contribute to the denominator. Expressing incidence as TB incidents per 100 herd-years at risk aims to take better account of the opportunity for infection to be detected.
2.2.2 Different Incidence Measures
Two other incidence measures are commonly used to determine TB incidence: New incidents detected ‘per 100 unrestricted herds tested (UHT)’ and ‘per 100 live herds tested’. Historically, the simpler approach of looking at the proportion of all herds with a TB incident in a year was used. This is also referred to as incidents per 100 live herds tested. This is derived from the number of new infected herd incidents per 100 herds tested during the period. Incidence per 100 live herds tested excludes herds that were not tested, and so in which TB is very unlikely to be found. However, it does not account for the different frequency of testing in each area of England. It does not account for the proportion of unrestricted herds that are at risk of a new TB incident either. All herds are thus included in the denominator, but only those tested are included in the numerator. The effect of testing some herds less than once a year means they have less potential to contribute cases to annual incidence calculation. This creates bias in the calculation and underestimates the rate at which new cases occur.
Using the number of unrestricted herds tested (UHT) in a year as a denominator addresses this issue to some extent and is the basis for calculating incidence per 100 UHT. In this case, the denominator time at risk is calculated as the time herds were without restrictions but only for the year of interest, rather than accumulated as when calculating the time at risk for incidence per 100 HYR. For example, in incidence per 100 UHT, a herd with no previous history of TB under a single set of restrictions between 1 September and 31 October of a year would contribute time at risk up from 1 January until 31 of August and then again from 1 November to 31 December. However, the incidence rate per 100 UHT remains dependent on the proportion of the herd tested overall. TB incidents detected through slaughterhouse surveillance also contribute to the numerator, but not to the denominator. As a result, TB incidents per 100 UHT tend to give the highest values of herd incidence compared with the other 2 methods. The 3 different incidence measures tend to give similar temporal patterns. However, the rate per 100 herd-years at risk is usually higher due to the smaller denominator.
2.2.3 Incidence Rate Ratios
Incidence rate ratios (IRR) quantifies the difference in disease incidence between different categories of animal or herd. This is the difference between herd size, herd type and risk area, relative to a reference category. An explanation of the choice of reference category is provided in Section 4.5. For example, an IRR of 2.0 means the incidence rate of TB in herds within that category was twice as high as the reference category. An IRR of less than 1 represents categories where the incidence rate is lower than that of the reference category.
To investigate the effect of herd type, Poisson regression was used to produce IRRs that adjusted for the effects of herd size and risk area. The adjusted IRRs for the herd size categories and risk regions were very similar to the unadjusted IRRs. As expected, the incidence rate was significantly lower in the Edge Area and LRA compared to HRA, despite adjusting for herd size and type.
2.2.4 Prevalence
Prevalence describes the proportion of herds in an area that are infected at a given point in time. This report presents point prevalence, measured by calculating the proportion of herds under restriction (due to a TB incident) at a given moment. Monthly point prevalence is the number of active herds under restriction divided by the reported number of active herds in the middle of each month. Annual average prevalence is the average of the 12 months within the year. At county level, the prevalence is the number of herds under restrictions divided by the number of total herds at the end of each year.
Herds restricted due to an overdue test rather than a TB incident are not classified as ‘restricted’ in this report. Therefore, estimates of the proportion of herds under restriction will be lower in this report than in the official TB statistics.
2.2.5 Recurrent TB incidents
A recurrent incident is defined as:
- a herd that had a TB incident disclosed in the reporting year
- and has also been under movement restrictions for a different TB incident in the previous 36 months
Recurrence can result from a general increase in incidence. For example, a herd would have a greater probability of a previous TB incident if the past incidence were high.
Recurrence likelihood can be increased if some herds are more likely to have repeated TB incidents than others for particular reasons relating to those herds.
The ‘current period’ refers to the reporting year (2021). The ‘history period’ refers to:
- the 36 months preceding the start date of the incident in the current period, or
- where no recurrent incident has occurred in a herd, is the 36 months prior to the mid-point of the current period
Analyses included all herds that were considered ‘live’ in the current period (2021), meaning active at the end of it. Whether the herd was live in the history period (preceding 36 months) was not checked.
Herds under restriction for 4 months or more in 2021 from a TB incident that started in the history period were excluded from analyses. These herds had limited opportunity to become TB incidents since there may have been no further testing in the period following their closure. This 4 month threshold helps to detect any recurrence in herds where restrictions were lifted within the first 4 months of the reporting year.
In recent years, recurrence has been described in terms of the relative risk (RR). This compares herds historically under movement restrictions with a new TB incident in the current year, to herds with no history of restrictions. This is then stratified by risk area, herd type and herd size. The RR divides the herds with a recent history of TB which had an incident in the current year, by those without a recent history of TB, but which also had an incident.
Using this method invites comparisons between the relative risks. However, at every level of each factor the risk to the denominator population is quite different, so comparisons are not strictly valid. This is particularly true for the differences across risk regions. It is also likely to be the case for herd size categories and herd types, which may also be confounded with another.
Based on these assumptions, it was agreed to recalculate this table using a logistic regression where the outcome would be the odds ratio (OR). This is the odds of a herd with a history of TB having another incident this year, compared to a herd with no history. The odds ratio (OR) is a measure of association between an exposure and an outcome. The logistic regression was run on each variable and ‘previous TB incident’ was used as an interaction term. This was to determine if the odds of having a TB incident in the current year were increased where a herd had a history of TB incidents.
Although recurrence is calculable from TB incident data, its cause in any given TB incident is difficult to discern. With surveillance data it is difficult to distinguish between persistent undisclosed (‘residual’) cattle infection from a previous TB incident and a newly introduced infection event.
2.2.6 Spatial extent of endemic TB
To identify the areas of England and Wales that are affected by endemic TB, a definition of endemicity was developed under the Defra-funded research project SE3045. This enables the expansion and retraction of the endemic TB area of GB to be measured over time. It also provides a useful tool for decision makers when reviewing the efficacy and implementation of local TB control measures. A geographical unit is considered endemic if there are at least 3 OTF-W incidents within a 5km radius within a 2 year period. The geographical unit used to map the expansion and retraction of the endemic area is a 500x500m grid cell.
This definition was developed through analysis of TB surveillance data and with input from APHA veterinary field staff. It is the most appropriate definition that can be applied on a national level. It is not perfect and will apply better in some areas than in others. For example, small ‘endemic’ TB areas can appear which could be temporary artefacts due to the chosen definition of endemicity. Whilst acknowledging the limitations of this definition, it does provide a generally applicable and reproducible approach for determining the endemic area. The definition may need to be refined in future to reflect changes in the epidemiology of TB over time and to changes to surveillance regimes.
2.2.7 Statistical methods
- All statistical calculations were performed in Stata v15.0.
- A chi-squared test was performed for comparing years, e.g. number of TB incidents in 2020 and 2021. A Fishers Exact test was used if a cell value was less than 5.
- The estimated significance probability for the Fisher’s Exact test for 2 x 2 tables with large numbers is taken from Pezzullo (2010). This is generally taken as a 2-tailed value.
- A z-test was used to compare prevalence between 2020 and 2021.
- Incidents rates were compared by analysing the deviation of the incidence rate ratio from 1, using the 2-tailed significance value.
- The median duration of TB incidents was compared using the K-sample equality-of-means test.
- The reference category chosen for categorical predictors in regression analyses varied. Ideally the reference category was both biologically relevant and had a sufficient number of observations or cases to be statistically sound.
However, if: - the most biologically relevant category had insufficient observations/cases, or - there was no clear biological advantage in selecting a reference category,
Then the category with the most observations/cases was chosen.
In some of the analyses performed, the number of TB incidents may vary depending on when data extraction and analysis were carried out. These variations are generally minor.
2.2.8 Risk pathway assessment
Assessing how a herd became infected with TB can be challenging. TB is a chronic, insidious infection, usually with a long incubation period. This means that in most animals clinical signs are only apparent when the disease is fairly advanced. TB is generally disclosed through skin testing (proactive surveillance), on farm or post-mortem surveillance at the slaughterhouse. The detection of TB may occur sometime after it arrives in a herd. As such, the evidence to retrospectively establish the source and routes of infection for an affected herd (the ‘risk pathway’) can be difficult to reconstruct. However, clarifying the risk pathway facilitates the use of targeted on-farm biosecurity measures to reduce the risk of reinfection for that herd.
TB incidents (both OTF-W and OTF-S) are investigated to assess the hazard (source of infection) and risk pathway. These investigations follow the set protocol described below. A ‘provisional assessment’ is made early on during the management of an incident, to help guide and prioritise immediate actions. A ‘final assessment’ is then repeated when all evidence has been gathered, including e.g. post mortem, tracing and culture results. The same protocol is used for both provisional and final assessments. However, the final assessment has 2 added categories, for the rare cases where infection was ultimately judged not to have been present. These are ‘non-specific reaction’ and ‘anomalous result’. Refresher training is provided to investigating officers to ensure that, as far as possible, the protocol for risk pathway assessment is applied consistently. In the HRA one third of new incidents are randomly selected for an investigation. The aim is to investigate all new incidents in the Edge Area and LRA. However, in some instances in the Edge Area resource constraints exist such that it is not be possible to investigate all breakdowns. Where this is the case, as many new incidents as possible are randomly selected or triaged for an investigation visit.
Protocol for risk pathway assessment
The investigating officer assesses all the evidence in order to identify the likely route by which TB infection entered the holding. Several plausible risk pathways are usually identified. The protocol asks the investigator to use all available evidence plus veterinary judgement, to describe how likely each of the risk pathways are. Up to 3 risk pathways can be recorded, and each must be scored using ‘definite’, ‘most likely’, ‘likely’ and ‘possible’. The investigator must summarise the evidence in support of their selections. They are advised that ‘although it will often not be possible on a particular farm to say for certain how the cattle got infected, consideration of how this may have happened in light of the husbandry practised, biosecurity measures in place, and other findings in the investigation will make some pathways more likely than others.’
Each assessment is comprised of 2 components. The first is a ‘hazard’, which is the original source of infection (for example infected cattle, badgers, other domestic or wild animals). The second is a risk pathway (for example cattle movements from a defined risk area or exposure during housing or at pasture). Additionally, investigators are asked to record risk pathways that have been excluded (such as movements on a closed farm or contiguous contact with no neighbouring cattle).
The following table shows how the 28 combinations of hazards and risk pathways selected by investigating officers in 2020 were aggregated. Nine sources of infection were identified and used to present the results of the risk pathway analysis at risk area and county level.
| Hazard | Risk pathway | Source of infection |
|---|---|---|
| Infected Badgers | Exposure at grazing, where all feed at grazing is inaccessible to badgers | Badgers |
| Infected Badgers | Exposure at grazing, where feed at grazing is accessible to badgers | Badgers |
| Infected Badgers | Exposure at housing, where all feed is inaccessible to badgers | Badgers |
| Infected Badgers | Exposure at housing, where feed stores are accessible to badgers | Badgers |
| Infected Cattle | Movements from Edge | Cattle movement |
| Infected Cattle | Movements from High TB Area Wales | Cattle movement |
| Infected Cattle | Movements from HRA | Cattle movement |
| Infected Cattle | Movements from Intermediate TB Area Wales | Cattle movement |
| Infected Cattle | Movements from Low TB Area Wales | Cattle movement |
| Infected Cattle | Movements from LRA | Cattle movement |
| Infected Cattle | Movements from NI or other country (imports) | Cattle movement |
| Infected Cattle | Movements from Scotland | Cattle movement |
| Infected Cattle | Contiguous over the fence or straying | Contiguous infection |
| Infected Cattle | Residual infection in the herd | Residual infection |
| Infected Domestic Animals | Co-located | Domestic animals |
| Infected Domestic Animals | Contiguous | Domestic animals |
| Anomalous Result | Anomalous Result | Non-specific reactor |
| Non-specific reaction | Mycobacterium other than M. bovis, or false positive result | Non-specific reactor |
| Uninfected | No Pathway | Non-specific reactor |
| Fomites, undetermined source | Contaminated cattle slurry or manure | Fomite source |
| Fomites, undetermined source | Contaminated purchased feed or bedding | Fomite source |
| Fomites, undetermined source | Contaminated Vehicles | Fomite source |
| Fomites | Other | Fomite source |
| Fomites, undetermined source | Shared equipment or machinery | Fomite source |
| Other Infected Wild Animals | Other | Other wildlife |
| Other Infected Wild Animals | Wild Boar | Other wildlife |
| Other Infected Wild Animals | Wild Deer | Other wildlife |
| Other or Unknown | Rare sources (must specify) or unknown (must explain logic) | Other or unknown |
Combining risk pathways and certainty
During the assessment, the APHA case veterinarian selects up to 3 possible risk pathways of infection for each TB incident herd. Each risk pathway is given a score that reflects the likelihood of that pathway being the true one. The weighting applied to the certainty score has been updated in 2019 to reflect the developing understanding of how likelihood is being assessed in practice. It is as follows:
- Definite - score 8
- Most likely - score 6
- Likely - score 4
- Possible - score 1
Any combination of definite, most likely, likely or possible contributes towards the overall picture for possible routes of introduction into a herd (see example 1). If the total herd score is less than 6, then the score is made up to 6 using the ‘Other/Unknown Source’ option (see example 2). Buffering up to 6 in this way helps to reflect the uncertainty in assessments where only ‘likely’ or ‘possible’ sources are identified.
Example 1
| Source of infection | Certainty | Certainty score | Weighted contribution |
|---|---|---|---|
| Cattle movement | Most likely | 6 | 0.55 |
| Contiguous cattle | Likely | 4 | 0.36 |
| Badgers | Possible | 1 | 0.09 |
| Total herd score | 11 | 1 |
Example 2
| Source of infection | Certainty | Certainty score |
|---|---|---|
| Infected cattle HRA | Possible | 1 |
| Infected cattle LRA | Possible | 1 |
| Other or unknown | Possible | 1 |
| Total herd score | 3 |
The total herd score is 3. As this is less than 6, ‘Other or unknown’ gets increased to 4 points, so that the total score is 6. Thus, the final weighted contribution of each risk pathway is as follows:
| Source of infection | Certainty | Certainty score | Weighted contribution |
|---|---|---|---|
| Infected cattle HRA | Possible | 1 | 0.17 |
| Infected cattle LRA | Possible | 1 | 0.17 |
| Other or unknown | Possible | 4* | 0.67 |
| Total herd score | - | 6 | 1 |
Interpreting source of infection outputs
The source of infection outputs combines the data from multiple herds. This provides the proportion of pathways in which each source was identified, weighted by certainty, as described above. The outputs do not show the proportion of herds where each pathway was identified (as this is skewed by the certainty calculation). The relative proportions of each risk pathway are approximations and only broad generalisations should be made from these data. Where a greater proportion of OTF-S herds are investigated there will be more uncertainly in the risk pathways, as genotyping evidence is not available.
3. Overview of TB testing in Great Britain
3.1 Measuring test accuracy
Sensitivity is the ability of the test to identify diseased animals. Specificity is the test’s ability to correctly measure that an animal is not infected. Both directly affect how well the control measures that seek to monitor infection moving out of and in herds will work. Such control measures include removing infected animals for the herd (removal of reactors), or to allow only uninfected animals to be moved (pre-movement tests).
Ideally, tests should have both high sensitivity and specificity, as effective disease eradication is dependent on finding and removing all infected animals. But a trade-off between sensitivity and specificity is often the norm. Lowering the threshold of a positive result to increase sensitivity increases the chances of wrongly categorising an uninfected animal as infected (meaning this reduces specificity).
Therefore, different test interpretation policies are applied according to the area, herd history and other factors, in order to make the best compromise for the circumstances. For TB, tests tend to be less sensitive, and as a result some infected animals may still give a negative test result. However, they tend to have a high specificity, so it is very unlikely that an uninfected animal will give a positive test result.
An important use of sensitivity and specificity values is to estimate a test’s predictive values. These determine test result outcome for the animal (positive or negative) and how accurate that result truly is.
The positive predictive value (PPV) of a test is defined as the probability that a positive testing animal is truly infected. Conversely, the negative predictive value (NPV) is the probability that an animal with a negative test result is truly free from infection. Both measures depend on the proportion of the population that is infected (prevalence of infection) as well as the sensitivity and specificity of the test.
The higher the prevalence of infection in a population, the higher the PPV and the lower the NPV of a diagnostic test. In other words, the same test for TB infection in cattle will not have the same predictive value when used in different risk areas. Both the stage of infection and disease prevalence in different risk areas have an effect on the TB diagnostic tests. Therefore, it is not easy to calculate ‘average’ predictive values for the diagnostic TB tests. However, these averages in areas of different prevalence can still be useful in helping plan how tests should be interpreted.
The single intradermal comparative cervical test (SICCT) is the main detection test used for surveillance in UK. It is strongly specific (meaning if cattle test positive, they almost certainly have TB), but can miss infected cattle. When this happens, some cattle may have no reaction, whilst others will not give a big enough reaction to be classified as positive. These are called ‘inconclusive reactors’ and will require an additional test to decide their true disease status. If they retest as inconclusive or positive, they are classified as infected and are slaughtered, and incident procedures are triggered.
The limitations of the SICCT can be addressed by changing the way its results are interpreted. This is done with the ‘standard’ or ‘severe’ interpretation, which changes the threshold of a positive result. It can also be improved by assessing the likelihood that IRs are truly infected, based on statistics or the use of the more sensitive gamma interferon test. These options are explored below.
The specificity of the SICCT test at different interpretations in GB was estimated by Goodchild et al in 2016. SICCT specificity was found to vary not only with the different positive cut-off points for standard and severe interpretation, but also across regions within GB. Table 5.1 shows how likely a positive animal is to be truly infected and how likely it is to have been exposed to infection.
According to the calculated PPV of the SICCT test, 91.8% of reactors in GB are infected. PPV values ranged from 92.3% in the high-prevalence counties to 76.9% in the low-prevalence counties. The study indicates that the SICCT test, as used in GB, has a very high specificity. Thus, at standard interpretation it will give rise to one false positive animal for every 4,760 to 7,690 animals tested. Conversely, the findings suggest that over 90% of reactor cattle identified only by skin test in GB between 2002 and 2008 were infected. This endorses the compulsory slaughter of all SICCT test reactor cattle for effective disease control.
Table 5.1. Selected data from Table 6 in Goodchild et al (2016):
Calculation of the PPV for the SICCT test in 3 groups of Great Britain counties that vary in TB prevalence. The 95% confidence intervals were based on the confidence intervals of specificity.
| Group of counties and description | High prevalence: England High-Risk Area +4 Welsh counties | Medium prevalence: England Edge Area +3 Welsh counties | Low prevalence: England Low-Risk Area + Gwynedd + Anglesey + Scotland | All of GB |
|---|---|---|---|---|
| PPV if the interpretation was severe only for OTF-W incidents (with 95% confidence interval) | 92.3% (91.1 to 93.7%) | 88.6% (86.4 to 90.9%) | 76.9% (72.1 to 82.0%) | 91.8% (90.5 to 93.3%) |
| PPV if all tests had been at severe interpretation (with 95% confidence interval) | 89.5% (88.2 to 91.3%) | 74.8%(71.6 to 79.0%) | 46.6 (39.7 to 55.4%) | 87.7 (86.1 to 89.7%) |
| PPV if all tests had been at ultra-severe interpretation (with 95% confidence interval) | 88.9% (87.4 to 90.3%) | 74.3 (70.7 to 77.5%) | 43.2 (35.3 to 50.3%) | 86.9 (85.1 to 88.6%) |
In summary, in high or intermediate prevalence situations, nearly every single reactor detected by the SICCT is truly infected. This is particularly the case in the HRA, or for short interval and herd risk tests. Thus, a positive SICCT test provides strong evidence of infection in TB incidents in these risk areas independent of post-mortem confirmatory evidence. As expected, PPV increases with the test sensitivity, specificity and animal prevalence.
However, the lower predictive value of the SICCT in lower incidence areas can detect positive animals which are not truly positive for infection with TB.
This has been the case especially in places such as the LRA, parts of Wales and in Scotland.
Note that measurement of all test characteristics depends on knowing the true status of the animal, which should be measured using a ‘gold standard’ test. However, such a test is lacking for cattle in the early stages of TB infection. The difficulty in knowing whether an animal is truly uninfected or merely asymptomatic makes estimating the specificity and NPV particularly challenging. Thus, careful use of test application or interpretation can help to improve predictive values. Consideration of other evidence is also important in deciding whether a negative animal is truly uninfected. Together, these enhance accuracy and enable the heterogeneity of the epidemic to be taken into account when designing control measures.
Actions to increase detection of infected cattle in a TB infected herd
Surveillance tests are imperfect, hence different options have been developed to enhance the chance of detecting all TB infected cattle in a herd. These include:
Increasing the number of herd SICCT tests (so called short-interval (SI) tests) that the herd must pass to regain OTF status following disclosure of one or more reactors.
Severe interpretation of skin test: Increasing the sensitivity in this way will reduce the specificity of the skin test. This means 1 in 1,111 cattle could potentially be a false positive reactor, instead of 1 in 5,000 at standard reading (see Strategy for achieving Official Bovine Tuberculosis Free Status for England). However, these values apply to individual animals. Test sensitivity is higher at herd level and classification of a herd as infected is more accurate, particularly when more than one reactor is detected.
Consider removal of non-reactor cattle as DCs: This relates to cattle that have been in contact with other infected cattle but can also be based on test history. For example, one or more previous classification as an IR, or belonging to a cohort where a high percentage of reactors have been detected.
Supplementing the skin test with the interferon-gamma blood test: Applying a parallel interpretation of the results so that animals reacting to either or both tests are regarded as infected and removed from the herd.
3.2 Types of surveillance used for detecting TB
3.2.1 Surveillance overview
Bovine tuberculosis is a slowly progressing and insidious disease that is not clinically apparent for some time after infection, but which can spread during this time. This means that surveillance on apparently healthy animals is needed in addition to investigating apparent clinical cases of TB, to get ahead of disease spread. Surveillance involves active surveillance where live animals are tested at set intervals. Active surveillance can be modified in the different parts of the country to reflect the different likelihood of TB either being present or being detected. It also requires passive surveillance, where there is a requirement for the government to be notified if anyone suspects TB infection in animals. The latter occurs mainly from cattle being processed through slaughterhouses.
Detecting TB with a diagnostic test depends on how likely it is for the disease to be present and how robust the test is. The likelihood of infection with TB is associated with location, herd type and size, as well as whether the herd has previously been infected. These important differences in likelihood of disease presence in herds affect how surveillance is best carried out and how effective it is at detecting infection.
3.2.2 Surveillance Stream definitions
The term ‘Surveillance Stream’ was coined in 2016 to classify bovine TB surveillance systems in the England Epidemiology Report. It was designed according to the intention behind the activities carried out to detect disease. Broadly speaking, there are 2 types of activities among the 4 streams defined below: slaughterhouse surveillance and application of the TB skin test (SICCT). The definitions for the surveillance streams encompassing these are below. Tests carried out as part of the Area & Herd risk surveillance stream have been further divided by purpose: primarily aiming to detect disease (surveillance) or aiming also to stamp it out (control).
Routine animal testing and slaughterhouse surveillance help to detect TB in herds or animals not expected to be at any increased likelihood of being infected. Area and Herd Risk surveillance and Proactive surveillance are targeted at herds or animals thought to be at higher risk of being infected. These types of surveillance also limit the impact of the movement of unknowingly infected animals to a lower risk area.
Surveillance stream definitions are as follows:
Routine: Disease first disclosed as a result of tests scheduled as part of routine surveillance with no expectation of increased or decreased probability of disclosing infection.
Area & Herd Risk: Disease first disclosed as a result of targeted tests carried out due to history. Alternatively, disease disclosed from tests carried out due to epidemiological evidence that there is a higher probability of disease in the animal or herd.
Slaughterhouse surveillance: Disease first disclosed as a result of routine post-mortem meat inspection during commercial slaughter of animals not believed at higher likelihood of being diseased. This excludes results of inspection of test reactor cattle and DCs removed by APHA.
Trade & Other surveillance: Disease first disclosed as a result of testing scheduled due to the high impact of disease if present, in the destination herd / premises. This includes tests on animals where the presence of some epidemiological risk factors may increase disease probability but is not the primary reason for testing. Referred to as ‘Proactive surveillance’ in earlier reports.
Table 6.2.1 Surveillance Streams classification
| Test Code | Name | England Surveillance Report Categories (2018 onwards) | Surveillance Streams (2016-2017) | Surveillance Purpose |
|---|---|---|---|---|
| CON, CON6, CON12 | Contiguous Test | Area & Herd Risk; Contiguous tests | Area & Herd Risk | Control |
| CT(RTA) | Check Test (Road Traffic Accident) | N/A | Area & Herd Risk | Surveillance |
| CT-HS1, CT-HS2 | Check Test (Hotspot) | Area & Herd Risk; Hotspot tests | Area & Herd Risk | Surveillance |
| RAD,RAD6, RAD12 | Radial Test | Area & Herd Risk; Radial test | Area & Herd Risk | Control |
| 90D | See TBU test | N/A | Area & Herd Risk | Surveillance |
| CT | Check Test | N/A | Area & Herd Risk | Surveillance |
| CT(EM) | Check Test (Exposure Mitigation) (Backward tracing herd test) | Area & Herd Risk; Source tracing test | Area & Herd Risk | Control |
| CT(I-I) | Check Test (Investigation and Intervention) | Area & Herd Risk; Check test | Area & Herd Risk | Surveillance |
| DTG | Delayed Testing Group | N/A | Area & Herd Risk | Control |
| IFN, IFN LOW IN, IFN PERSI, IFN NSR, IFN SLHERD, IFN ANOM, IFN NBCP/PBCP, IFN OTH_SP, IFN PRI | Gamma Interferon Test (OTF-W LRA, OTF-W persistent TB incidents, non-specific reactor herd, whole or partial reactor herd slaughter, anomalous reactions procedure, badger culling area tests, disease in other species present, private | Area and Herd Risk; Not typically disclosing tests | Area & Herd Risk | Control |
| PSI | Partial Short Interval Test | N/A | Area & Herd Risk | Control |
| SI | Short Interval Test | Area and Herd Risk; Not typically a disclosing test | Area & Herd Risk | Control |
| IASI | SI test in OTF-S herds in Wales Intensive Action Area only | N/A | Area & Herd Risk | Control |
| TBU (former 90D) | TB Unit Test (AFUs) | Area and Herd Risk; AFU test | Area & Herd Risk | Surveillance |
| 12M | Twelve Month (Post-TB incident) Test | Area and Herd Risk; Post-incident tests | Area & Herd Risk | Control |
| 6M | Six Month (Post-TB incident) Test | Area and Herd Risk; Post-incident tests | Area & Herd Risk | Control |
| IR/IFN 2xIR | Inconclusive Reactor Test / Gamma Interferon Test severe 2xIRs (Wales only) | Area and Herd Risk Not typically a disclosing test | Area & Herd Risk | Surveillance |
| TR | Traced Bovine Test | Area and Herd Risk; Spread tracing tests | Area & Herd Risk | Control |
| Old RH* | Reformed Herd Test | N/A | Area & Herd Risk | Surveillance |
| ASG | Approved Segregated Group Test | Area and Herd Risk; Not typically a disclosing test | Area & Herd Risk | Control (if on TB incident herd) |
| AI | Artificial Insemination Centre Test | Trade and Other Pre-movement testing | Trade & other | Surveillance |
| BHH | Bull Hirer Test (Scotland only) | N/A | Trade & other | Surveillance |
| CT-LRA-SA | LRA Pre-Sale Check Test | Trade and Other; Pre-sale check LRA | Trade & other | Surveillance |
| EX | Export Test | Trade and Other; Pre-export | Trade & other | Surveillance |
| PII | Post-Irish Import Test | Trade and Other; Post-export | Trade & other | Surveillance |
| PIO | Post-Import Test | Trade and Other; Post-export | Trade & other | Surveillance |
| POSTMT/ POSTMOVNC/ POSTMOVOV | Post-Movement Tests | Trade and Other; Post-movement | Trade & other | Surveillance |
| PRI | Private Test | Trade and Other; Private tests | Trade & other | Surveillance |
| PRMT | Pre-Movement Test | Trade and Other; Pre-movement testing | Trade & other | Surveillance |
| CT-NH1/2/3 | New Herd Test | Routine; New Herd tests | Routine | Surveillance |
| OT | Other | N/A | Routine | Surveillance |
| RHT/RHT48 | Routine Herd Test | Routine; Routine Herd tests | Routine | Surveillance |
| WHT | Whole Herd Test | Routine; Whole Herd tests | Routine | Surveillance |
| IA6/IA12 | Routine 6M/12M test within the Welsh Intensive Action Area area only | N/A | Routine | Surveillance |
| SL | Slaughterhouse | Slaughterhouse Surveillance Stream | Slaughterhouse Surveillance Stream | Slaughterhouse Surveillance Stream |
3.3 Tools used to support TB incident analysis
3.3.1 Genotyping
Attempts are made to recover M. bovis from all TB incidents and to subject at least one isolate per TB incident to molecular (DNA) typing. This identifies the ‘genotype’ (a sequence of numbers and letters) of the M. bovis isolate. Further analysing its spoligotype and VNTR (Variable Number Tandem Repeat) helps identify particular sequences in the genome of the bacterium. This knowledge is used to describe areas where particular genotypes are common, so-called ‘home ranges’, and to compare isolates from new TB incidents with the previous known distribution, including the home range, of the particular genotype identified. From 19 April 2021, Whole Genome Sequencing (WGS) started to replace genotyping (spoligotype and VNTR type) as the predominant technique used to genetically classify M. bovis isolates and genotyping was switched off in December 2021.
3.3.2 Whole Genome Sequencing
As of 19 April 2021, all incident herds with M. bovis positive culture results started to have Whole Genome Sequencing (WGS) results recorded instead of genotyping results. Despite being relatively novel, WGS is the current gold standard method for strain differentiation, giving a higher level of differentiation between isolates and providing insight into their evolutionary relationships.
WGS provides information on the entire genome of M. bovis. This contrasts to the previous genotyping methods used (a combination of VNTR or Spoligotyping as explained in section 3.3.1), which target specific regions of the bacterial genome. WGS therefore achieves higher resolution of M. bovis strains compared to conventional genotyping as it allows for finer-scale differences across the entire DNA sequence to be identified. WGS examines variation caused by mutations across the entire DNA sequence of the M. bovis genome (4.4 million base pairs in length) and therefore offers higher discriminatory power compared to conventional genotyping for differentiation of M. bovis strains.
Because WGS analyses the whole genome, identical or very similar genome sequences can only be obtained from closely genetically related isolates. In contrast, genotyping of isolates means that identical or similar repeat patterns are not necessarily genetically linked, as they can evolve independently.
WGS identified 6 major groups (B1-B6) which were subdivided into a total of 30 clades. Each isolate is assigned to a WGS clade based on its genome sequence. The WGS clade names consist of the letter ‘B’ for M. bovis followed by a number (for example, B4, B6 etc.) that indicates which major group the isolate belongs to. Further numbers (for example, B6-13, B6-42) indicate the subgroup within the major group to which the isolate is assigned.
The clades assigned to isolates are designed to be hierarchal: For instance, B6-42 is more closely related to B6-41 than it is to B6-13; and B6-41, B6-42 and B6-13 are more closely related to each other than to B3-11. Due to the slow mutating nature of M. bovis, isolates from multiple breakdowns assigned to the same clade are more likely to be linked.
WGS is an important tool used by APHA for investigating TB breakdowns and possible transmission pathways between cattle herds. At farm level, WGS helps APHA field vets to identify the most likely source of TB infection for a breakdown herd, and also whether it has spread to other cattle herds. Once APHA is aware of the likely origin of a breakdown, they can advise farmers on measures they could take to reduce the risk of further infection entering the herd. WGS is also routinely applied to M. bovis isolates from non-bovines including South American camelids (alpacas, llamas), goats, pigs, and captive deer when investigating TB incidents affecting these species, as well as in wildlife species (for example, road killed badgers) and compare them with isolates from local TB breakdowns in cattle herds and other farmed animals. This allows APHA to assess whether cattle and badgers from the same geographical area are affected by the same strain of the TB bacterium, and to assess the spread of TB strains between geographical areas and across time. For instance, WGS is being used to support TB surveillance in cattle and badgers and understand the epidemiology of TB in the confirmed hotspot area in east Cumbria (HS21), where a link between cattle and badger TB infection was first identified in 2017.
3.3.3 Homerange maps
Homerange maps are an attempt to capture the geographical localisation (endemic regions) of the various WGS clades of M. bovis found in GB. They have proved extremely useful for identifying the expected WGS clade at a given location, and support epidemiological investigations at national, regional and individual incident level.
A homerange defines a geographical area in which a certain WGS clade of M. bovis is not unexpected. A simple algorithm to define the homerange area was originally developed as part of Defra research project SE3257. This is the same criterion as that used in previous reports to the European Commission. A 5km square is considered part of a certain homerange if:
- there have been at least 3 different incidents of that WGS clade
- on at least 2 holdings
- from 3 or more discrete years within a 5 year window
In order to create a coherent area for each genotype, a 10km buffer is then drawn around each of the homeranges so defined.
3.3.4 Slaughterhouse Performance Model
The model was set up to explore patterns in residual variation in detection rates between slaughterhouses after accounting for different animal level risk factors. These were: sex, age, breed, days in high/low risk herds, contact with high/low risk herds, surveillance testing status, year, quarter, and risk area. Estimations of the likelihood that a slaughterhouse will detect a TB infected animal during routine slaughter according to which animals it processes, can then be carried out. These patterns can be summarised by the posterior mean odds ratio (OR). Slaughterhouses with positive posterior ORs are detecting more tuberculous carcases at commercial slaughter of non-reactor cattle than the average expected. Slaughterhouses with negative posterior ORs are finding fewer than the average expected. However, by design it would be expected that the ORs for the different slaughterhouses to be distributed above-and-below the average. Thus, due to the large heterogeneity in throughputs, the ORs for some slaughterhouses will be better estimated than others.
The perceived reliability of this estimation is measured by the posterior variance. Those slaughterhouses with a lower variance are likely to have a more accurate estimation of the posterior mean. By considering the 95% credible interval around the posterior mean OR using the variance, the slaughterhouse’s performance can be statistically estimated. If this interval includes zero, then the model does not provide sufficient evidence that the slaughterhouse is detecting any more or fewer cases than expected. If the interval is entirely below zero, then the model suggests that the slaughterhouse is detecting significantly fewer cases than expected given the other factors. Equally, if the interval is entirely above zero then the model is suggesting that the slaughterhouse is detecting significantly more cases than expected. The size of the interval is mainly down to the throughput of the slaughterhouse. The higher the throughput, the narrower the interval, and hence the more precisely the mean is estimated.
4. Policies for TB control in Great Britain
4.1 TB control in England
Bovine TB is statutorily controlled in England following The Strategy for achieving Officially Bovine Tuberculosis Free status for England (2014) and the Bovine TB Strategy Review (2018). The aim of the strategy is to eradicate TB by 2038, whilst maintaining an economically sustainable cattle industry. Under the strategy, England is divided into 3 areas reflecting the level of disease in each and controls differ accordingly (Figure 2). Control in all areas is based on a range of surveillance and control measures. Up to date information on current policies can be found at www.tbhub.co.uk.
Figure 2. TB risk and surveillance areas of England effective since January 2018, as set out in the Government’s Strategy for Achieving Officially Tuberculosis-Free Status for England.

The high risk area is in the south-west and mid-west of England. The low risk area is in the south-east, mid-east and north of England. They are separated by the Edge area. The Edge area adjacent to the high risk area has been eligible for 6 monthly testing since January 2018. The 2 most northern counties of the high risk area have been eligible for 6 monthly testing from September 2020.
4.1.1 Summary of control measures by risk area in England
Summary of controls in the High Risk Area
Surveillance controls:
- slaughterhouse surveillance
- annual or 6 monthly herd testing (see earned recognition below)
- compulsory pre-movement testing
Incident management:
- within herd - movement restrictions, isolation, slaughter and compensation, epidemiological investigation, tracing, additional skin tests and IFN-γ blood testing
- additional skin testing in neighbouring herds
Reduce risk of TB from badgers:
- licensed injectable badger vaccination
- licensed badger culling
Other disease prevention:
- biosecurity measures
- risk-based trading
Summary of controls in the Edge Area
Surveillance controls:
- slaughterhouse surveillance
- annual or 6 monthly herd testing (see earned recognition below)
- compulsory pre-movement testing
Incident management:
- within herd - movement restrictions, isolation, slaughter and compensation, epidemiological investigation, tracing, additional skin tests and IFN-γ blood testing
- additional skin testing in neighbouring herds
- additional skin testing in herds within a 3km radius (in annual herd testing areas only)
Reduce risk of TB from badgers:
- licensed injectable badger vaccination
- licensed badger culling
Other disease prevention:
- biosecurity measures
- risk-based trading
Summary of controls in the Low Risk Area
Surveillance controls:
- slaughterhouse surveillance
- four-yearly testing (annual for high risk herds)
- pre-sale check tests
- compulsory post-movement testing for cattle from the annual or 6 monthly surveillance areas
Incident management:
- within herd - movement restrictions, isolation, slaughter and compensation, epidemiological investigation, tracing, additional skin tests and IFN-γ blood testing
- additional skin testing in herds within a 3km radius
Reduce risk of TB from badgers:
- licensed injectable badger vaccination
- licensed badger culling (in exceptional circumstances)
Other disease prevention:
- biosecurity measures
- risk-based trading
Earned Recognition
Since May 2019, cattle herds in the 6-monthly testing areas that meet certain criteria have been eligible to return to annual surveillance testing through earned recognition. These criteria are either:
- the herd has been in existence for at least 6 years and has not had a TB incident in that 6 year period
- the herd is registered to a bovine TB health scheme accredited under the Cattle Health Certification Standards (CHeCS) at level one or above
4.1.2 New TB control measures introduced in England in 2021
Changes to the gamma testing policy in the HRA and Edge Area of England: Previously, gamma testing was mandatory for all new TB breakdowns with lesion or culture positive animals, and in persistent breakdown herds, in the LRA and Edge Area. In the HRA, gamma testing was mandatory for all new TB breakdowns with lesion and/or culture positive animals where either:
- the APHA veterinary investigation concludes that the most likely bTB transmission route for the affected herd was contact with infected cattle and measures are in place to limit further spread of disease
- the infected herd is in one of the areas where at least 2 annual rounds of effective licensed badger culling have been completed.
- there is clear evidence that repeat skin testing of the herd has failed to resolve a TB breakdown, meaning chronic and persistent breakdowns
A new policy for mandatory interferon-gamma testing of TB incident (breakdown) herds in England came into force in July 2021. Mandatory interferon-gamma blood testing now applies to:
- new TB breakdowns with lesion or culture positive animals occurring within 18 months of the conclusion of a previous breakdown with lesion or culture positive animals in the same herd (HRA/Edge areas on 6-monthly surveillance testing regimen)
- all new breakdowns with lesion or culture positive animals in the LRA and annual surveillance testing parts of the Edge Are
- chronic and persistent breakdowns with lesion or culture positive animals anywhere in England
Changes to 6-monthly surveillance testing in the HRA: From July 2021, default 6 monthly testing was rolled out across the rest of the HRA, having been first introduced in Staffordshire and Shropshire in 2020. Cattle herds at lower risk of a TB breakdown have the option to remain on annual surveillance testing, if they meet one of the ‘earned recognition’ criteria.
The Tuberculosis in Animals (England) Order 2021: Coming into force on 1 October 2021, this new Order incorporated the existing statutory controls for TB in domestic cattle, farmed Asiatic water buffalo, farmed bison and non-bovine farmed animals in England, and replaced the Tuberculosis (England) Order 2014 and the Tuberculosis (Deer and Camelid) (England) Order 2014.
CHECS TB Entry Level Membership: In summer 2021, CHECS launched a new TB Entry Level Membership for cattle farmers. It provided a baseline biosecurity standard, comprising of a range of easily achievable biosecurity measures recognised by the British Cattle Veterinary Association (BCVA), Defra and the Welsh Government to reduce the risk of a TB breakdown in herds.
Changes to movements on and from AFUs/AFUEs: From July 2021, cattle from AFUs in England and Wales and AFUEs in England were allowed to move to and be sold at approved TB dedicated sales (“Orange Markets”) in England and Wales.
TB Advisory Service (TBAS) Successor contract: The TB Advisory Service was extended to all areas of England (including the LRA), to cover cattle farmers as well as keepers of farmed non-bovine animals.
Discontinuing COVID-19 temporary amendments to TB skin testing in Great Britain: In the summer of 2021, the temporary easements of the skin testing rules in GB due to the COVID-19 epidemic came to an end as follows:
- Suspending APHA referrals of overdue TB skin tests in GB for COVID-19 reasons (ended 1 July 2021).
- Extending the short interval testing window by 30 days in England and Wales (ended 1 July 2021).
- Exempting calves under 180 days old from certain routine and targeted TB skin tests in England and Wales (ended 1 August 2021).
Badger vaccination: Badger vaccination was carried out between 1 May and 30 November 2021, across a licenced area of 474.92km2; 159.69km2; in the HRA, 174.31km2; in the Edge Area and 140.92km2; in the LRA.
Licensed badger culling: Seven new Badger Control Programme (BCP) areas were licensed by Natural England in 2021. Three of those areas were introduced in the HRA and 4 were in the Edge, bringing the total number of active BCP areas in 2021 to 40. In addition, a total of 21 areas undertook licensed Supplementary Badger Control in 2021 (See Chapter 3.4, TB control in wildlife).
4.2 TB control in Scotland
Scotland has been officially bovine TB (TB) free (OTF) since 2009. The Scottish Government is committed to a comprehensive, practical and proportionate programme of actions to maintain current low levels of TB and safeguard OTF status.
TB controls in Scotland are underpinned by the Tuberculosis (Scotland) Order 2007 and by the risk based routine herd testing policy introduced in 2012. These policies meant “low risk” herds became exempt from the 4 yearly routine herd testing programme.
Further information on TB in Scotland can be found on the Scottish Government website.
No new measures came into effect in Scotland in 2021.
4.2.1 Previous TB cattle measures in Scotland introduced before 2021
On the 12th December 2018 the Tuberculosis (Miscellaneous Amendments) (No 2) Order 2018 came into force.
These new rules include changes to disease control measures and compensation arrangements.
4.3 TB control in Wales
Bovine tuberculosis (TB) is subject to statutory control in Wales and is directed by the principles set out in the Bovine TB eradication programme. TB controls are underpinned by the Tuberculosis (Wales) Order 2010. Details about TB in Wales and the surveillance and control measures associated with the eradication programme can be found on the Welsh Government website.
New TB policy measures introduced in Wales in 2021
Additional measures introduced in the Low TB Area (LTBA) and Intermediate TB Area North (ITBAN) areas in Wales: From the 1st of November 2021, certain regions in the LTBA will be temporarily incorporated into the ITBAN region. Other measures introduced include additional testing and free Cymorth TB “Keep it out” visits in herds contiguous to an OTF-W incident.
New culture and WGS arrangements following post-mortem examination: New measures for culture and WGS of bovine TB from slaughtered cattle introduced in 2021.
Non-validated testing and Enferplex pilot protocols: New pilot to request use of the non-validated Enferplex Bovine TB antibody test was opened to the public in July 2021.
Changes to movements on and from AFUs/AFUEs: From January 2021, cattle from AFUs in England and Wales and AFUEs in England were allowed to move to and be sold at approved TB dedicated sales (“Orange Markets”) in the High TB Area East (HTBAE) region in Wales.
Previous TB policy measures introduced in Wales before 2021
Removal of inconclusive reactors (IRs) (from January 2020): Change in policy from the removal of all standard and severe inconclusive reactors in a persistent herd breakdown, to only removing standard IRs and IFN-γ and IDEXX testing severe IRs.
IDEXX change (from January 2020): As part of the exit strategy for persistent herd breakdowns, when only severe IRs are identified in a short interval test, these animals are tested by IFN-γ and if positive, they are all IDEXX tested. If the IFN-γ test comes back all negative, then this is considered a clear test.
Exclusion of calves under 180 days old from routine or targeted herd surveillance (from April 2020): calves under 180 days could be temporarily excluded from a surveillance test.
From May 2020, calves under 180 days could be temporarily excluded from breakdown testing, but not from breakdown clearing tests. These measures were put in place in response to the COVID-19 emergency and lasted through the year.
Temporary suspension of overdue referrals: in March 2020, overdue referrals were temporally suspended, but herds were restricted. This lasted through 2020 and led to more herds being under restrictions as tests were overdue.
Glossary
Animal and Plant Health Agency (APHA): APHA was founded 1 October 2014. It merged the former Animal Health, Veterinary Laboratories Agency, Plant and Bee Health, GM Inspectorates, and the Plant Varieties and Seeds Office. This created a single agency responsible for animal, plant and bee health in Great Britain.
Annualised: Conversion of a variable into a yearly sum (for example, by multiplying a quarterly incidence by 4).
Approved Finishing Unit (AFU): A type of TB unit approved by APHA providing an outlet for the finishing of negative-testing cattle from multiple TB restricted and unrestricted herds. LFUs are permanently under TB movement restrictions (TB02), and cattle are housed under biosecure conditions. Cattle in the unit are exempt from routine TB testing. Animals may be sourced from multiple unrestricted premises in any area of England, Scotland and Wales. AFUs without grazing are located in the HRA and Edge Area in England, and the High TB Areas in Wales.
Approved Finishing Unit -Enhanced (AFUE): A new type of approved finishing unit with grazing providing an outlet for the finishing of negative-testing cattle from multiple TB restricted and unrestricted herds. Animals must have 2 consecutive negative skin tests before being allowed out to graze on the holding. Animals are on the AFUE are tested at regularly 90 day intervals until slaughter. AFUEs are in operation in the High Risk Area (HRA) of England only.
Bovine tuberculosis (TB): Disease of cattle and other mammals caused by infection with Mycobacterium bovis
Breakdown: See ‘TB incident’.
Case: See ‘TB incident’.
Compensation: The statutory payment made by the competent authority to the owner of the animals that have been culled for TB eradication purposes. There are different statutory compensation systems for cattle slaughtered in England, Scotland and Wales.
Contiguous herd: Strictly speaking, a holding that has a common boundary with the TB incident holding of interest, but includes herds separated only by a short distance (for example, across a road or river, or where an epidemiological assessment indicates there is likely to be risk of exposure to infection.
Dangerous contact (DC): A non-reactor animal in an OTF-W TB incident herd considered to be at such high risk of being infected that slaughter is justified. Usually for the reason of contact with infected cattle.
Disclosing test: The test that triggers the start of a new TB incident which in turn marks the start of movement restrictions. Includes TB incidents disclosed through a confirmed slaughterhouse case.
Earned recognition (ER): A scheme that allows lower risk herd in 6-monthly routine surveillance areas to qualify for annual testing, if they meet certain conditions
Eradication Programme: A programme aimed at achieving biological extinction of an animal disease or zoonosis and/or to obtain the free or officially TB free status of the territory.
Fomites: Objects or materials which could potentially carry and infectious agent, such as purchased feed or bedding, vehicles or shared equipment or machinery - more commonly a problem in other animal diseases (for example AI, FMD, Salmonella).
Gamma interferon test (IFN-γ or IFN-g or gIFN or IFN-gamma): A laboratory-based blood test that is approved as an ancillary diagnostic tool. The test measures the amount of the cytokine (immunological messenger molecule) IFN-γ released in whole blood cultures stimulated with specific antigen. It is used to supplement the skin test in certain TB incident herds, rather than as a standalone test.
Genotype: A unique DNA type or ‘strain’ of Mycobacterium bovis, defined by a combination of spoligotype (expressed as a number) and VNTR type (expressed as a letter). This information is used to characterise the molecular epidemiology of the TB bacterium in GB. It supports APHA epidemiological investigations into the origin of individual TB incidents.
Herd: An animal or group of animals kept on a holding as an epidemiological unit. In GB they are identified with a County Parish Holding Herd (CPHH) number.
Herd size: For a TB incident, herd size is the largest size entered into SAM (see ‘SAM’) for a test conducted at any time during the incident. For officially TB free herds, herd size has been changed in 2017. Median size is now recorded on the BCMS Cattle Tracing Scheme for the holding over the most recent 12 months with a recorded size. For holdings with more than one herd, or not present in BCMS, the herd size at the most recent whole herd test was recorded. Where no size is retrievable from either source the typical number of animals indicated on SAM has been used. The drive to change to using CTS was to reduce the numbers of both those without a retrievable size from the testing history and those where recent tests presented no eligible stock.
Herd test: A surveillance or control test triggered by a herd-level event. In contrast to a test triggered for an individual animal or a small group of animals within a herd.
Herd types: ‘Beef’ includes Beef, Suckler, Beef Heifer Rearer, Beef Bull Hirer, Beef dealer, Stores herds and Meat Buffalo herds; ‘Beef fattener’/ ‘Beef finisher’ includes beef finishing herds; ‘Dairy’ includes Dairy, Dairy Dealer, Dairy Bull Hirer, Dairy Producer, Dairy Heifer Rearer, Producer Buffalo and Domestic herds; ‘Other’ includes Calf Rearers, unspecified Dealer Herds, Artificial Insemination (AI), Bison and herds described on SAM as ‘Other herds’.
Herd-years at risk (HYR): The sum of the time (in days, months or years) that herds in the geographical area of interest are unrestricted. Herds that have received a herd-level test during the period of interest are at risk of a new incident.
Holding: A holding is a place where livestock, including cattle, are kept or handled in pursuit of an agricultural activity. It may be a farm, or other premises such as a market, lairage, abattoir or showground. Some keepers may have more than one holding, and some holdings may be used by more than one keeper. A holding is not the same as a business. It is expressed as a County Parish Holding (CPH number) and a single holding may comprise of one or more herds.
Homerange: The geographical area in which a particular WGS clade of M. bovis is typically recovered from infected cattle herds. A 5km square is considered as part of a certain homerange if there have been 3 different incidents of that clade, on at least 2 holdings, within a 5 year window. To create coherent areas for each clade, a 10km buffer is then drawn around each of the homeranges so defined.
Incidence: The incidence of a disease is the rate at which new cases occur in a defined population over a designated time period.
Inconclusive reactor (IR): An animal showing a particular pattern of reactions to a comparative intradermal tuberculin test. The difference in size of reactions to bovine and avian tuberculin is not large enough to cause it to be described as a reactor. Such animals are usually isolated and subjected to a second skin test after 60 days. However, they can be removed earlier as DCs (see ‘DC’), IFN-γ test reactors (see ‘Gamma interferon test’), or voluntarily slaughtered by their owner.
Inter-quartile range (IQR): A measure of statistical dispersion equal to the difference between the upper and lower quartiles: (meaning the 75th and 25th percentiles of the distribution’s values).
Licensed Finishing Unit (LFU): A type of TB unit approved by APHA providing an outlet for the finishing of negative-testing cattle from multiple officially TB free (OTF) herds. LFUs are permanently under TB movement restrictions (TB02), and cattle are housed under biosecure conditions. Cattle in the unit are exempt from post-movement TB testing, providing the statutory pre-movement testing requirements have been met. Animals may be sourced from multiple unrestricted premises in any area of England, Scotland and Wales. LFUs are present in the Low Risk Area (LRA) of England and the Low TB Area of Wales only.
Linear regression: A statistical approach for modelling the relationship between a continuous outcome variable (for example, duration of restrictions, which can take any value) and one or more ‘predictor’ variables (for example, herd size, herd type or county).
Live herd or Active herd: A herd of cattle, farmed buffalo or farmed bison defined in the CPHH notation which was “live” (meaning not archived), flagged as active on SAM on 31st December, 2020. This gives different values from the Agricultural Census, which is at holding level and updated at a different point in time.
Logistic regression: A statistical approach for modelling the relationship between a binary outcome variable (for example, positive or negative result) and one or more ‘predictor’ variables (for example, herd size, herd type or county).
Monitoring (programme): A programme to investigate an animal population or subpopulation, and/or its environment (including wild reservoir and vectors). This helps to detect changes in the occurrence and infection patterns of an animal disease or zoonosis.
Movement restrictions or restrictions: Legal prohibitions or restrictions on the free movement of animals into and out of a herd. Movement restrictions may be imposed on a herd because of the presence, or the suspected presence, of M. bovis infection. They may also be placed if statutory tests are overdue, leading to the loss of Officially TB Free herd status (see below). They can also be imposed if IRs are disclosed in a herd with a history of OTF-W incidents in the previous 3 years. Herd restrictions triggered by overdue tests are excluded from analyses in this report to avoid overestimates of disease.
Mycobacterium avium (M. avium): The causative organism of avian tuberculosis, which occasionally infects cattle.
Mycobacterium bovis (M. bovis): The causative organism of bovine tuberculosis.
New TB incident: A herd newly found to be infected with TB. Defined as a herd previously OTF in which at least one test reactor, IR taken as a reactor, or a culture-positive slaughterhouse case, has been found. The restriction, and thus the incident, starts on the disclosing test date (or date of slaughter of the slaughterhouse case). To qualify as being “new”, the incident must have been disclosed (meaning discovered) in the period specified in the report. The incident ends on the date the TB10 form is served and restrictions are lifted (see also ‘TB incident’ below).
Non-visible lesions (NVL): No lesions typical of bovine TB could be detected in the carcass at post mortem examination or meat inspection.
Officially bovine tuberculosis free status (OTF): A herd in which infection with M. bovis is not suspected and is able to trade animals freely. See WOAH Terrestrial Code Article 8.11.6 for a full definition of the officially TB free status.
Officially bovine tuberculosis free status suspended (OTF-S): For the purposes of this report, OTF-S is the status of a herd with a TB incident where there is suspicion of infection being present. A TB incident that did not meet the conditions for an OTF-W incident (see below) is classified as an OTF-S incident.
Officially bovine tuberculosis free status withdrawn (OTF-W): Refers to a herd with a TB incident where additional evidence of M. bovis infection has been identified in at least one slaughtered bovine animal. Thus M. bovis must have been identified in a cultured tissue sample and/or lesions detected in the carcass of a SICCT or IFN-γ test reactor. It also includes other incidents upgraded to OTF-W for epidemiological reasons.
Orange markets: Approved dedicated sales for TB-restricted cattle in the HRA and Edge Area of England.
Persistent herd or persistent TB incident herd: Refers to a TB incident herd that has been under restrictions for at least 550 days (approximately 18 months).
Poisson regression: A type of statistical modelling based on a particular type of numerical distribution. Used to compare rates of rare occurrences between different population groups, different areas, or different times.
Post-mortem or post-mortem examination: Examination (to various extents) of the carcass and organs of slaughtered cattle for lesions typical of bovine TB. Undertaken at an APHA Regional Laboratory, at the slaughterhouse following suspicion of infection (for example, reactors, IRs and DCs), or as part of routine meat inspection.
Pre-movement testing (PrMT or PRMT): Mandatory testing for cattle aged over 42 days old moving out of an at least annually tested herd into other herds. Also for cattle moved out of herds in the LRA to Scotland, unless the animal had spent its entire life in the LRA.
Prevalence: The prevalence of disease is the proportion of a defined population affected by that disease at a designated point in time.
Reactor (R): An animal showing a positive reaction result to a single intradermal tuberculin comparative cervical (SICCT) test, or to a gamma interferon (IFN-γ) assay. This is consistent with it being infected with M. bovis. This does not include an animal first suspected to have TB at the slaughterhouse. An animal that tests twice as inconclusive reactor to the SICCT test is automatically classified as a reactor. However, this will not count towards statistics for reactors throughout these reports.
Recurrent herd incident: A herd with a TB incident disclosed in the reporting year (2021) also under movement restrictions for a different TB incident in the previous 36 months.
Reference category (Ref): In regression analyses the reference group acts as a baseline against which other groups of interest are compared.
Reservoir: The reservoir is the population of animals in which the disease is circulating, and therefore is a common source of infection to other animals or humans.
Risk Area or Surveillance Risk Area or Surveillance Area or TB Area: Since 1 January 2013, TB testing intervals for bovines in England are 6-monthly, annual or 4-yearly at county level. The Strategy for achieving Officially Bovine Tuberculosis Free status for England (April 2014) set out 3 risk surveillance areas, which are followed in this report:
- High Risk area (HRA – annual routine surveillance testing)
- Edge area (annual or 6-monthly testing)
- Low Risk area (LRA – 4-yearly testing for most herds)
In 2017 Wales adopted a regionalised approach to TB distinguishing 5 TB areas as per the Wales Bovine TB Eradication Programme. These were Low TB Area, Intermediate TB Area North, Intermediate TB Area Mid, High TB Area West and High TB Area East.
SAM database (SAM or Sam): APHA’s IT system, which records, for example, details of herds, TB tests, TB incidents and the details and results of any tested and slaughtered cattle.
Sensitivity (of a test) (Se): The proportion of truly infected individuals in the screened population that are identified as infected (positive) by the test.
Severe interpretation: The positive cut-off criterion used to interpret the results of a skin test in TB incident herds to achieve a greater sensitivity. Using this interpretation of the SICCT, animals showing either:
- a positive bovine reaction and negative avian reaction or - a positive bovine reaction more than 2mm greater than a positive avian reaction are deemed reactors
Short Interval Test (SIT): See ‘Test code definitions’
Single intradermal comparative cervical test (SICCT): Also commonly referred to as the ‘skin test’ or ‘tuberculin skin test’. The test involves the simultaneous injection of a small amount of M. bovis and M. avium tuberculins into 2 sites on the animal’s neck skin. These are purified protein derivatives (PPD), a crude extract of bacterial cell wall antigens. Any swelling which develops at the injection sites after 72 hours are then comparatively measured to assess positivity (delayed-type hypersensitivity reaction).
Slaughterhouse (SLH) case: This refers to an incident whereby an animal from an OTF herd is found to have lesions consistent with TB during routine post-mortem meat inspection. In order that the case becomes an OTF-W incident, M. bovis must be isolated on culture from samples of the lesions. Until M. bovis is isolated at culture, a slaughterhouse case remains suspect and does not contribute to incident figures within this report. This is unless any subsequent skin check test performed in the herd of origin identifies reactors.
‘Smoothed’ or ‘12-month moving average’: A 12-month moving average is the average of the values for the current month and the previous 11 months. Moving averages can be any length. But, in general, shorter lengths will be best at identifying turning points and longer lengths best at identifying trends.
Specificity (of a test) (Sp): The proportion of truly uninfected individuals in the screened population that are identified as uninfected (negative) by the test.
Spoligotype: The result of one molecular technique used for genomic typing of organisms of the Mycobacterium tuberculosis complex, known as Spacer Oligonucleotide typing.
Standard deviation (SD): The standard deviation measures the spread of the data around the mean value. It is useful in comparing sets of data which may have the same mean but a different degree of variability in raw values.
Standard interpretation: The positive cut-off criterion normally used to interpret the results of a skin test. Using this interpretation of the SICCT, animals with a positive bovine reaction over 4mm greater than a negative or positive avian reaction are reactors.
Surveillance: Surveillance refers to activities to collect and record data on specific diseases in defined populations over a period of time. This helps to assess the epidemiological evolution of the diseases and the ability to take targeted measures for control and eradication.
Surveillance Streams Definitions:
- Area & Herd Risk: Disease first disclosed due to tests carried out in light of any evidence that there is a higher probability of disease in the animal / herd.
- Trade and Other: Disease first disclosed as a result of testing scheduled due to the high impact of disease if present in the destination herd / premises. Includes tests on animals where the presence of some epidemiological risk factors may increase disease probability but this is not the primary reason for testing.
- Routine: Disease first disclosed as a result of tests scheduled as part of routine surveillance with no expectation of increased or decreased probability of disclosing infection.
- Slaughterhouse (SLH) Surveillance: Disease first disclosed during routine post-mortem meat inspection of animals not believed at higher likelihood of being diseased. Excludes results of inspection of reactor cattle
TB area: There are 3 TB areas (High Risk Area, Edge Area and Low Risk Area) in England based on the distribution of TB within England.
TB Incident: A herd that has been categorised as infected with bovine tuberculosis is called a TB infected herd, and the event is called a ‘TB incident’. Also referred to as a ‘breakdown’ or ‘case’. The criteria that determine this are given under the definition of a ‘new TB incident’ above.
TB10 form or notice (TB10): A notice served at the end of a TB incident to lift the restrictions imposed on cattle movements onto and off the holding. It restores the OTF status of the herd.
Test code definition: Hotspot Test (HS): Carried out on herds within a confirmed TB Hotspot area in England. All bovines except calves under 42 days of age are included.
Test code definition: Private Test (PRI): A test carried out on individual animals. This is commissioned and paid for by the owner and carried out by an official veterinarian (OV) with the Regional Veterinary Lead (RVL) agreement. For example, extra TB test in a breeding bull.
Test code definition: Radial Test (RAD): Carried out on herds within a 3km radius of herds that have had their Officially Tuberculosis Free status withdrawn (OTF-W) in the Low Incidence Area or in the parts of the Edge Area on annual testing (England only). The initial RAD test is followed by a RAD6 test 6 months later and, in the LRA only, by a RAD12 test 12 months after the RAD6 test. All bovines except calves under 42 days of age are included.
Test code definition: Routine Herd Test (RHT): Routine surveillance herd test carried out in parishes with a 48 month testing interval. It must include:
- breeding bulls (for example, entire males over 12 months)
- females which have calved
- young bovines which will be used for breeding whether they are home-bred or purchased (except calves under 6 weeks old)
- pet cows and other non-commercial cattle resident on the holding
Test code definition: Short Interval Test (SI Test or SIT): Carried out in breakdown herds 60 days after removal (or effective isolation) of the last reactor or from a previous short interval test with no reactors. In England, they include all bovines except calves under 42 days old (unless there is an epidemiological risk of infection within that age group). They include all bovines in Scotland and Wales.
Test code definition: Whole Herd Test (WHT): Carried out routinely every 12 months in annual testing areas and in individual herds requiring annual testing, for example, producer-retailer dairy herds, bull hirers, heifer rearers, city or open farms, AI centres, and so on. It can also be carried out via RVL discretion in 48 month testing areas. It includes all bovines except calves under 6 weeks old.
Testing interval: Testing interval for routine TB surveillance purposes. In England the interval is either every 6 months, one or 4 years, depending on the policy applied to the risk area. In Scotland it has always been 4 years, and annual in Wales since 2010. Testing interval is given by the Area Testing Interval (ATI), Area Monitoring Regime (AMR), or the Unit Monitoring Regime (UMR) to which individual herds are allocated by APHA. In Wales, the ATI is recorded for the third quarter of the year in question, irrespective of whether the herd was tested in that year. Any shorter interval assigned specifically to an individual herd within a parish has not been used.
Time at risk (TAR): Time spent not under restriction since the most recent herd-level test. Alternatively, end of incident and time at risk of being diagnosed with TB during the observation period.
Tracing tests: Tests carried out to ‘trace’ the potential source or spread of infection.
- Backward’ tracings, also known as ‘source tracing’ tests investigate where infection may have originated, for example, the herd of origin of purchased cattle suspected of being infected when they arrived
- ‘Forward’ tracings, also known as ‘spread tracing’ tests, check individual animals that have left the herd when infection was believed to be present. This is to determine whether they are infected and may have carried infection to their destination herds
VetNet database (VetNet): VetNet is the predecessor of SAM, APHA’s TB control and surveillance system. Data was migrated into SAM from VetNet when SAM was launched in 2011.
Visible lesions (VL): Lesions typical of bovine TB detected in the carcass of a SICCT or IFN-γ test reactor at post-mortem examination or meat inspection.
Whole genome sequencing (WGS): Whole genome sequencing (WGS) replaced genotyping on 19 April 2021. WGS determines the entire DNA sequence of M. bovis isolates, allowing for characterisation of M. bovis strains at a higher resolution than genotyping. This ultimately allows a greater degree of certainty about the origin of TB infection for a herd and increases our understanding of how TB spreads both locally and nationally.
WGS clade: A group of phylogenetically (evolutionarily similar) related isolates based on similarities across their whole genome sequences.
