NCARDRS statistics 2016: summary report
Updated 29 September 2021
This document is a summary of a more detailed report produced by the National Congenital Anomaly and Rare Disease Registration Service (NCARDRS). It is NCARDRS’s second annual report and describes information about congenital anomalies detected in babies delivered between 1 January and 31 December 2016.
1. About NCARDRS
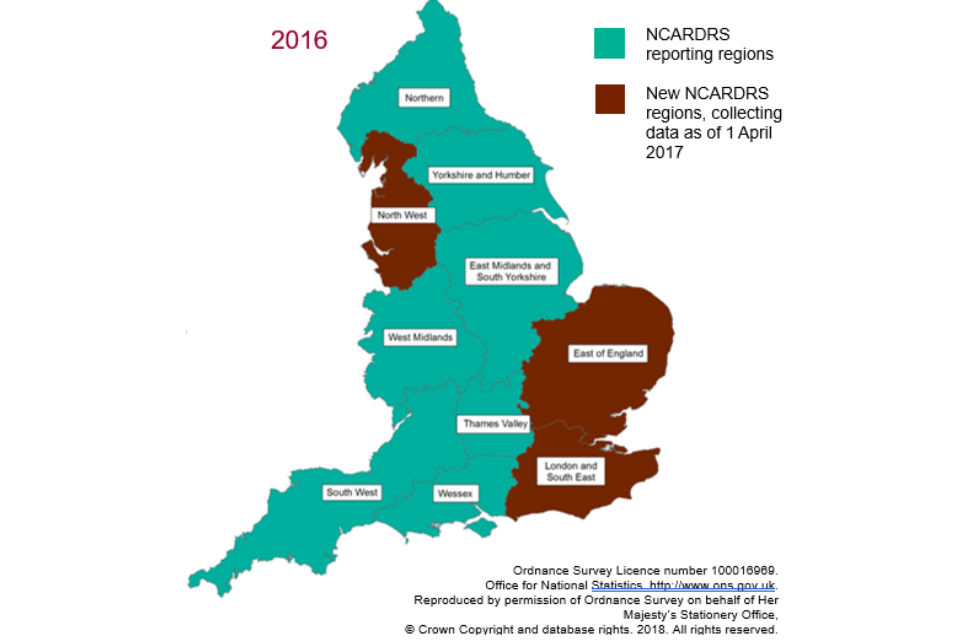
Public Health England launched NCARDRS on 1 April 2015. Prior to this, registries existed in some regions of England. In response to the UK Rare Disease Strategy and the Chief Medical Officer’s recommendation to ensure national coverage, 3 new regions covering the East of England, London and the South East, and the North West were established.
NCARDRS is made up of 10 regions, and information in this report is based on data collected from the 7 existing reporting regions.
Data collection in the newly established regions started from 1 April 2017. This means, for the first time, that national coverage of congenital anomaly reporting will be possible from 2019.
Our vision is to develop a comprehensive registration service that collects and quality assures data on congenital anomalies and rare diseases in England.
2. Prevalence of congenital anomalies
Congenital anomalies are defined as being present at delivery, probably originating before birth, and include structural, chromosomal, genetic and biochemical malformations[footnote 1].
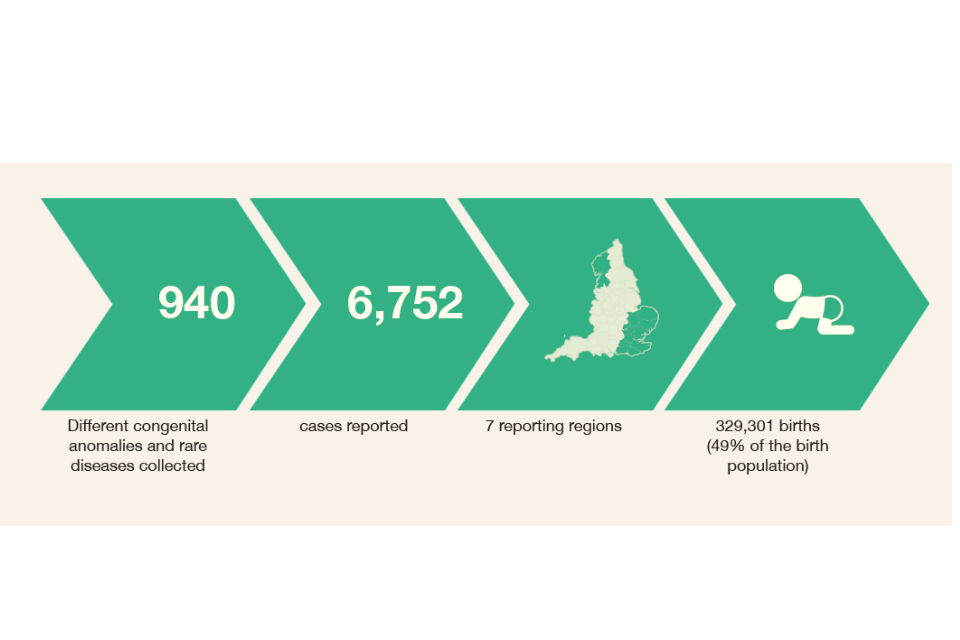
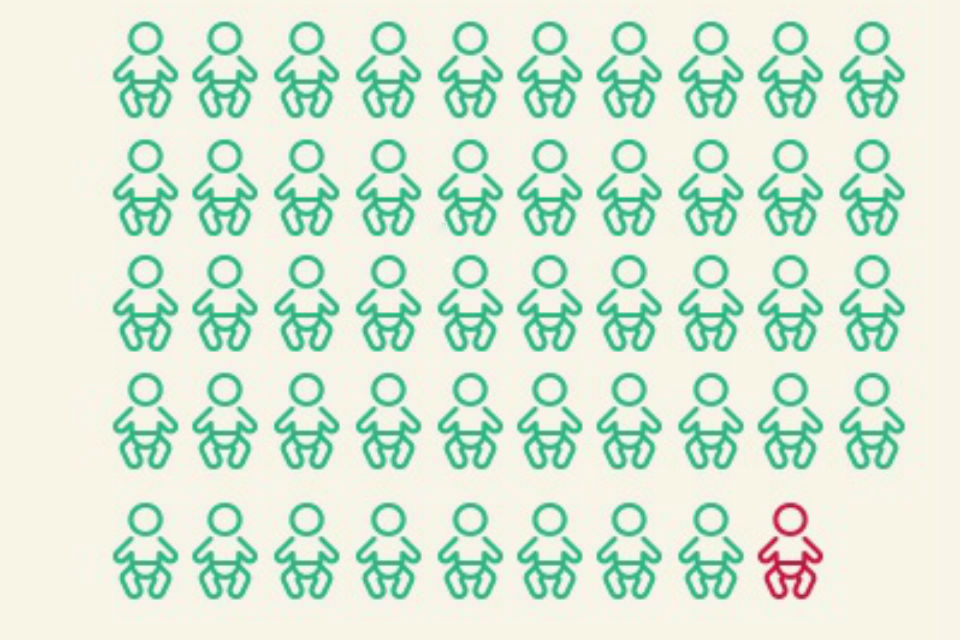
NCARDRS currently collects data about 940 different congenital anomalies and rare diseases. In 2016, there were a total of 6,752 cases with one or more congenital anomalies notified to the 7 NCARDRS reporting regions covering 329,301 total births (live births and stillbirths). This gives an overall birth prevalence for these regions of 205.0 per 10,000 total births – or 1 in 49.
3. Timing of diagnosis
Some congenital anomalies are detectable during pregnancy and others are not. Screening programmes are offered by NHS maternity services to maximise antenatal detection of specified conditions where women choose and present in time to have screening. In 2016, the timing of first diagnosis was known for 6,408 (94.9%) cases and of these, 64.9% were diagnosed antenatally.
Where a congenital anomaly was detected antenatally, 57.6% of cases resulted in a live birth, and where a congenital anomaly was diagnosed postnatally, 95.0% were diagnosed following a live birth.
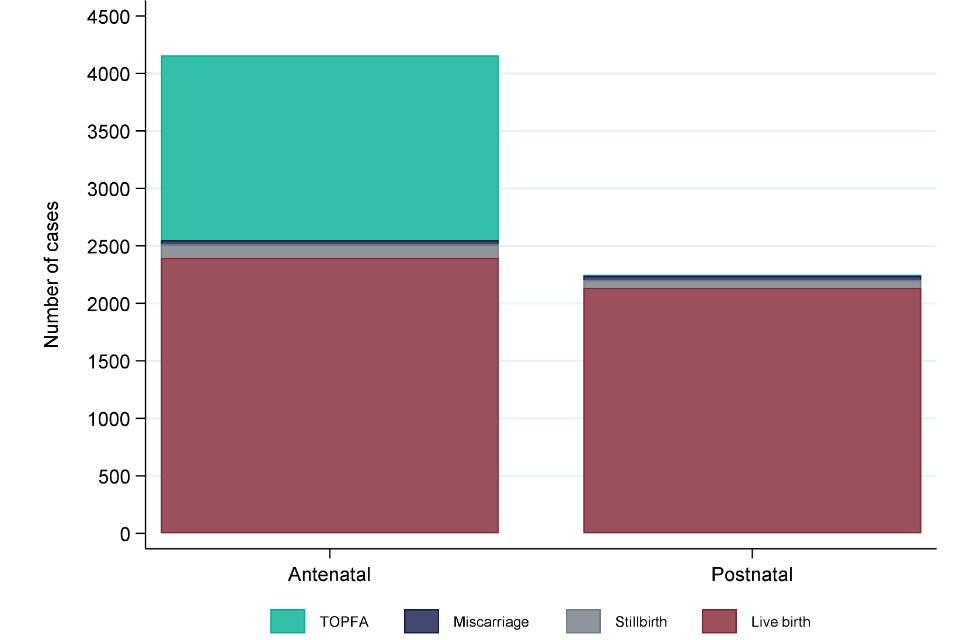
Figure 1: Timing of first diagnosis and pregnancy outcome in 7 NCARDRS reporting regions, 2016
4. Perinatal and infant mortality
Of the 6,752 congenital anomaly cases reported to NCARDRS, 336 resulted in an infant death, giving an infant mortality rate of 10.2 per 10,000 live births. Congenital heart anomalies accounted for 47% of infant deaths, followed by chromosomal anomalies (25%) and digestive system anomalies (18%).
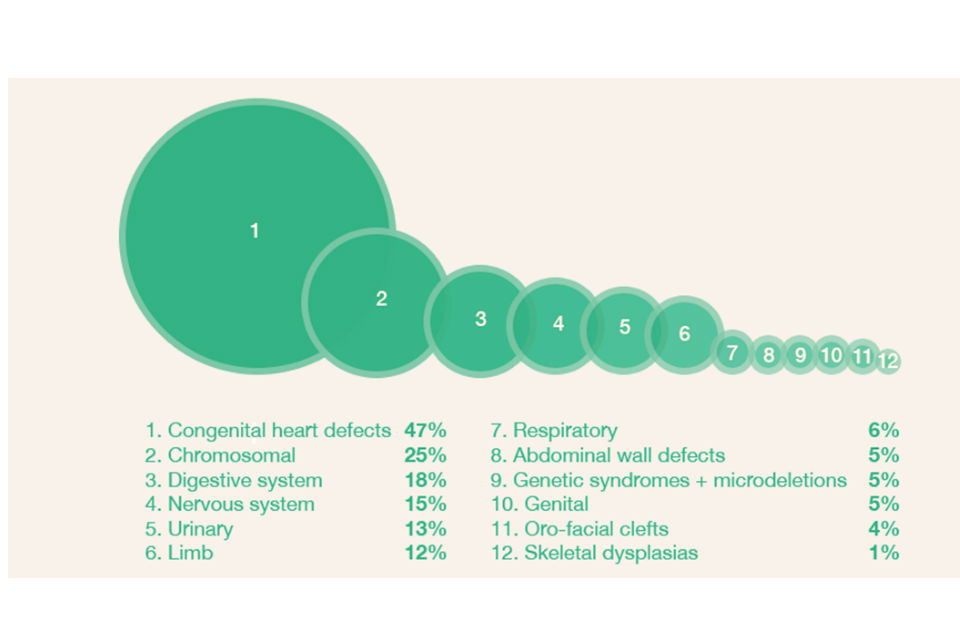
Figure 2: Perinatal mortality by congenital anomaly subgroup in 7 NCARDRS reporting regions, 2016
Data recorded in 2016 shows that the prevalence of genetic congenital anomalies increased with maternal age; the prevalence of genetic anomalies was 7 times higher in older mothers (women aged 40+) compared with younger mothers (women aged less than 20).
5. The NHS Fetal Anomaly Screening Programme
Eleven structural anomalies and Down’s syndrome are auditable under the NHS Fetal Anomaly Screening Programme (FASP). Where appropriate, antenatal screening is offered to women; the take-up of screening is entirely dependent on the choice of women and their families.
The data collected for the period 2015 to 2016, representing 33.8% coverage of NHS trusts providing antenatal services in England, indicates that detection targets were met for 6 of the 11 structural anomalies. Where the target was not met, this was statistically significant only in the case of Edwards’ syndrome. This was based on a small number of cases. We are currently collecting data with full national coverage in order to report detection rates for 2017 to 2018 data in 2019.
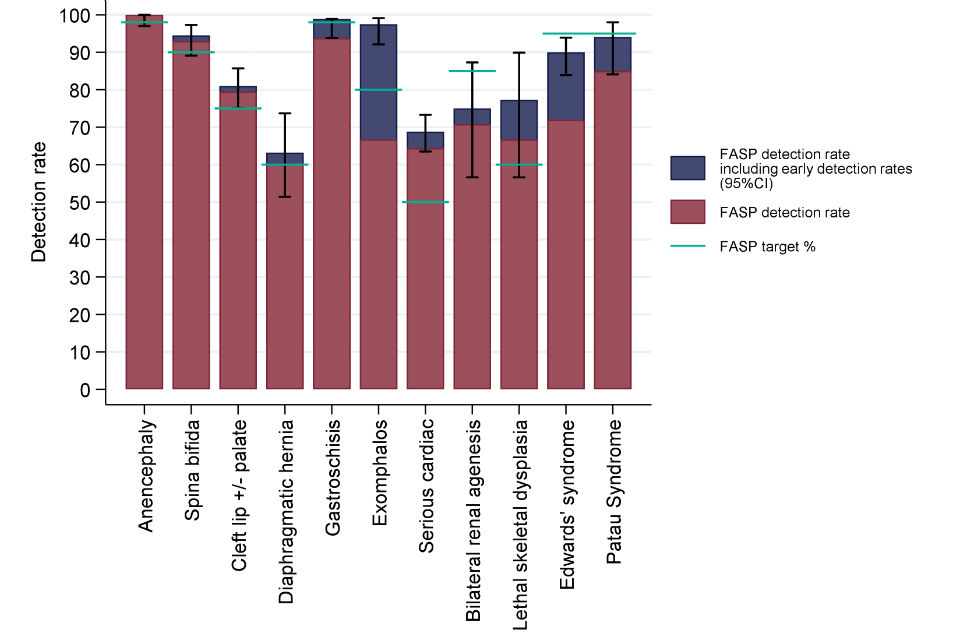
Figure 3: Detection rates, 95% confidence intervals and FASP targets by anomaly including early detections in 46 English reporting trusts, 2015 to 2016
6. Next steps for NCARDRS
This second annual report has demonstrated improvements over the past year towards achieving national coverage to enable robust information about the prevalence of congenital anomalies. NCARDRS looks forward to being able to present, for the first time in England, national coverage of congenital anomalies as at 1 April 2017 and being able to report on this data from 2019.
NCARDRS has been working to process and analyse congenital anomaly diagnostic data from cytogenetic laboratories recorded in 2015 and 2016. This work is nearing completion and NCARDRS plans to publish a national trisomy report in early 2019. This will provide updates to 2013 and 2014 data reported on Down’s syndrome, Edwards’ syndrome and Patau’s syndrome presented in the 2015 annual report. NCARDRS are currently preparing the 2016 to 2017 FASP audit data, and this will be reported to NHS trusts in September 2018.
There are plans in place to expand the national service to include rare disease data collection. NCARDRS is collaborating with experts, both national and international, to help prioritise conditions, understand more about collecting this data and how best to interpret and share findings. More detailed information about congenital anomalies and our current rare disease work can be found in the detailed report.
Finally, thanks must be given to the healthcare professionals dedicated to reporting cases to NCARDRS. NCARDRS also acknowledges the work and support of our expert scientific and clinical advisory panel.
Feedback on this report would be welcome; if you would like to get in touch, please send your comments and suggestions to ncardrs@phe.gov.uk.
