Edwards' syndrome screening quadruple test consultation
Published 21 February 2024
This consultation is open to the public and stakeholders (individuals and organisations) who are involved with and interested in antenatal screening.
Consultation question:
Does the evidence from this rapid review demonstrate that the quadruple test is accurate enough to be added to the antenatal screening pathway for Edwards’ syndrome (Trisomy 18)?
The consultation begins on Wednesday 21 February and ends at 5pm on Wednesday 6 March.
Consultation responses and enquiries should be emailed to uknsc@dhsc.gov.uk.
1. Introduction
The UK National Screening Committee (UK NSC) asked the NHS Fetal Anomaly Screening Programme (FASP) and the screening quality assurance service (SQAS) to determine the accuracy (detection rate and false positive rate) of the quadruple test to detect Edwards’ syndrome (Trisomy 18 or T18) using retrospective data from the English programme.
In January 2022, a paper was presented to the Fetal, Maternal and Child Health Reference Group (FMCH) summarising the modelling of the existing data on the detection of T18 using the quadruple test. The existing parameters of the NHS FASP screening gestational age window, and chance cut-off, were used. The FMCH generally accepted the paper, and the authors were asked to update the paper to include Patau’s syndrome (T13) along with T18 so that the quadruple test mirrors screening in the first trimester using the combined test.
This is the updated paper with the modelling data to include T13 as well as T18.
2. Background
In 2019, the Wolfson Institute of Preventive Medicine (WIPM) submitted a proposal to the UK NSC to modify the NHS FASP programme by using analytes from the quadruple test in the second trimester to screen for T18.
The quadruple test uses maternal age and 4 biochemical markers to calculate the chance of a baby having Down’s syndrome (T21). The test is offered within the NHS FASP programme between 14+2 and 20+0 weeks of pregnancy to those who miss, or do not complete, the first trimester combined test. There is no evidence that Inhibin levels differ between T18 and euploid pregnancies but alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG) and unconjugated oestriol (uE3) have lower levels in T18 pregnancies than in euploid pregnancies. This means that the quadruple test could be undertaken using chance results specific to T18. For T13, the evidence is that Inhibin is increased but, for other quadruple test markers, levels are not substantially different from euploid pregnancies.
The UK NSC commissioned an evidence review, which found one low quality systematic review and 13 moderate to high quality observational studies showing sensitivity was variable while specificity was consistently high across chance thresholds. When using a patient-specific chance threshold of 1 in 100, sensitivity was above 65% in most studies (ranging from 57.1% to 100%) and specificity was above 99%. However, there were applicability concerns as none of the studies were performed in the UK and none used the threshold of 1 in 150 as used in the UK.
There may be additional reasons to consider this proposal for earlier T18 and T13 detection and termination choice:
- this test would not be offered to the whole screening population of pregnant women, only a subset of women who miss their first trimester screening
- women do have the opportunity to undergo additional testing for T18 and T13 at their ultrasound scan, which may help compensate for the moderate test sensitivity
- a modelling study from WIPM using serum analyte samples from T18-affected pregnancies in the UK reported a triple test sensitivity of 57% and false positive rate (FPR) of 0.19% using a ≥1 in 150 risk threshold, which is consistent with data identified by this rapid review
3. Current FASP pathway
There are 3 points in current pathways where it is possible to detect T18 and T13:
-
Dating scan – the primary screening method for T18 and T13 in the first trimester is the combined test. A dating scan is a necessary component of the combined test; required to measure the crown rump length and nuchal translucency (NT). T18 and T13 may be detected by ultrasound at this point but it should be noted that there is no formal first trimester fetal anomaly ultrasound screening programme in England for T18 and T13.
-
Combined test – uses maternal age, NT and 2 biochemical markers to identify the chance of T21 and T18/T13. The test is offered within the NHS FASP programme between 10+0 and 14+1 weeks of pregnancy. The biochemical markers are free beta human chorionic gonadotropin (bhCG) and pregnancy associated plasma protein-A (PAPP-A). The combined test accounts for 86% of all tests within the NHS FASP programme. The detection rate (DR) for T18 exceeds the 80% target and the programme detected 89.4% (95% CI; 86.2-92.7%) of babies with T18 in 2019/20. The target detection rate (DR) for T13 is set at 80% and the programme detected 74.9% (95% CI; 67.5-82.3%) of babies with T13 in 2019/20 [footnote 1].
-
The 20-week screening scan – NHS FASP has a target DR of 95% for T18 and T13. Table 1 shows the DRs for T18 and T13 using data from NCARDRS for pregnancies with an expected date of delivery (EDD) 1 April 2019 to 31 March 2020.
Table 1: Detection rate for T18 and T13 2019-2020 EDD
| Condition | FASP target DR | DR 18+0 to 20+6 | DR 18+0 to 23+6 | Up to 23+0 inc early detections |
|---|---|---|---|---|
| T18 | 95% | 73.7% (CI 61.0-83.4) | 79.7% (67.7-88.0) | 97.7% |
| T13 | 95% | 91.7% DR (CI 74.2-97.7) | 92.0% (75.0-97.8) | 98.9% |
As shown in figures 1 and 2 below, when early detections are included the DRs exceed the 95% NHS FASP threshold.
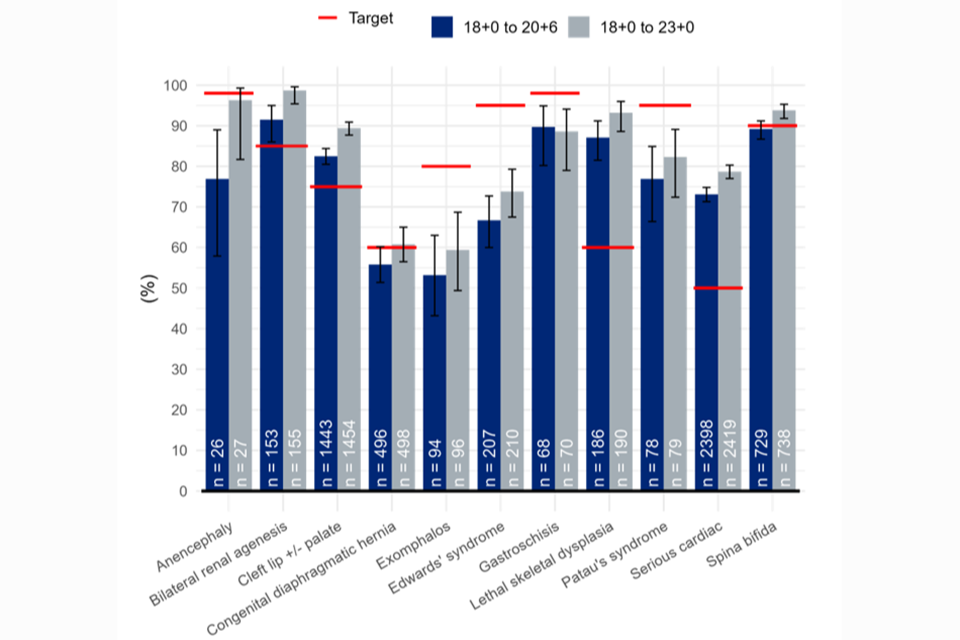
Figure 1: 18+0 to 20+6 week screening scan detection rates (%): conditions screened for as a minimum in England by gestational window, national, pooled EDD 2017/18 – 2019/20.
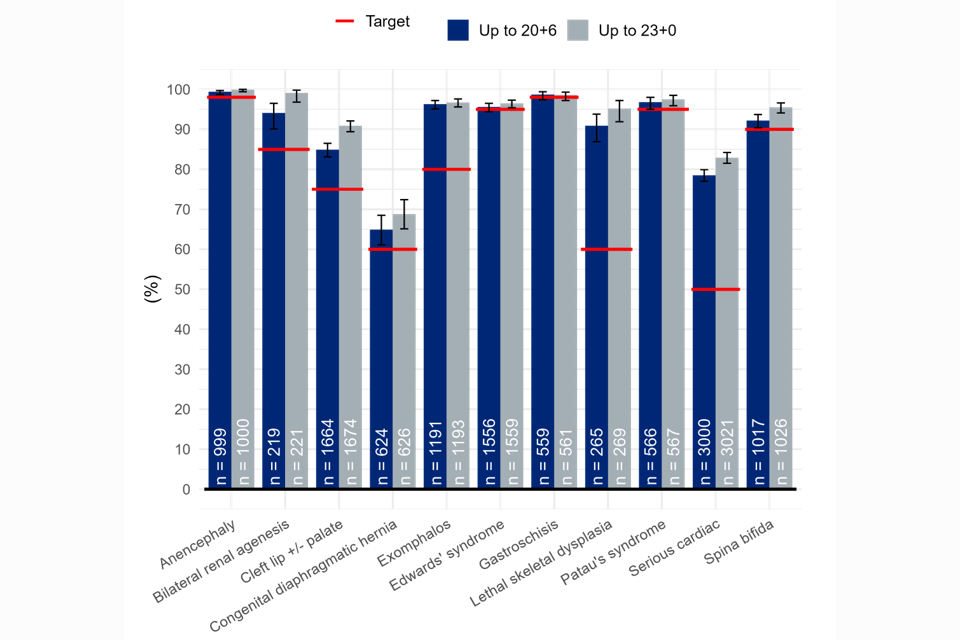
Figure 2: 18+0 to 20+6 week screening scan detection rates including early detections (%): conditions screened for as a minimum in England by gestational window, national, pooled EDD 2017/18 – 2019/20.
There is a proportion of babies with T18 and T13 who are undetected by the 20-week screening scan (Figures 3 and 4). Some women also booked too late to have a 20-week screening scan and some of these would also be too late to have the quadruple test.
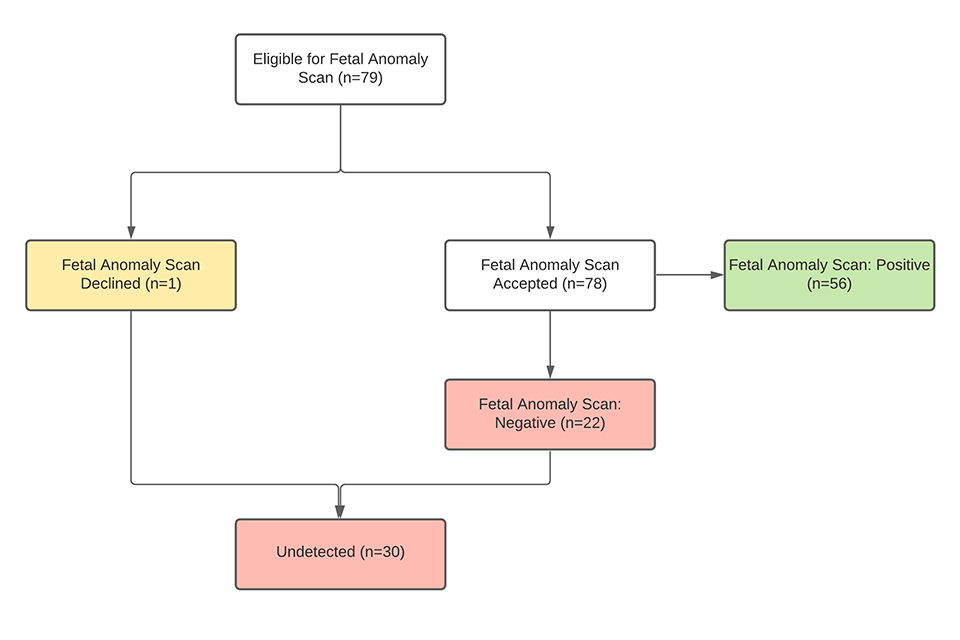
Figure 3: Babies with T18 who had a negative 20-week screening scan 2018/19 National Congenital Anomaly and Rare Diseases Registration Services (NCARDRS) data.
Seven women were too late to have the 20-week screening scan.
Of the 22 women with a negative 20-week scan:
- 7 declined the combined test
- 11 had a lower chance combined test result
- 1 booked too late for combined and had a quadruple test
- 3 had failed combined because the NT was not measurable, and all went on to have quadruple test
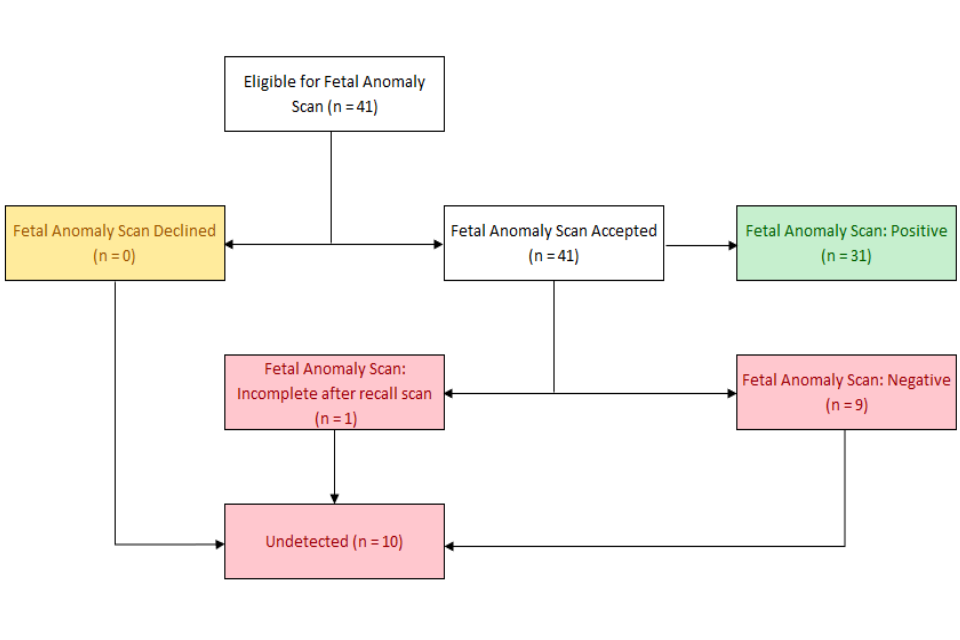
Figure 4: Babies with T13 who had a negative 20-week screening scan 2018/19 and 2019/2020 NCARDRS data.
Of the 9 women with a negative 20-week scan:
- 2 declined combined
- 2 booked too late for combined screening
- 5 had a lower chance combined result
4. The quadruple test population within the screening programme
For the 14% of women who for some reason are ineligible (for example present after 14+1 weeks of pregnancy) for combined testing, the second trimester quadruple test is offered for T21 but not for T18 or T13. The quadruple test population each year comprises around 70,000 women. Among these, 78 would be expected to have a T18 pregnancy and 25 would be expected to have a T13 pregnancy (Table 2).
| All | Combined | Quad | % | |
|---|---|---|---|---|
| Unaffected | 497,930 | 428,220 | 69,710 | 99.6% |
| Down’s syndrome | 1,334 | 1,147 | 187 | 0.3% |
| Edwards’ syndrome | 558 | 480 | 78 | 0.1% |
| Patau’s syndrome | 178 | 153 | 25 | 0.04% |
| Total | 500,000 | 430,000 | 70,000 | 100% |
| 86% | 14% |
Table 2: Modelled T21/T18/T13 screened population for NHS England
5. Performance of quadruple testing for T18
5.1 Methodology
Screening performance was obtained from a simulation model with distributional parameters taken from the literature [footnote 2]. Data were simulated from the multivariate Gaussian distribution of log transformed multiples of the median (MoM) values of AFP, uE3 and hCG in T13, T18 and T21 pregnancies. These were then used to compute likelihood ratios for T13, T18 and T21 relative to euploid pregnancies. Bayes theorem was used to compute chance results for T18 and T13 by combining the likelihoods for the biomarkers with the maternal age specific prior probabilities. These resultant chance results for T21 and T18/T13 were compared with cut-offs of 1 in 150 to determine an age specific DR for each year of maternal age from 12 to 50. The weighted average of these age specific rates was then computed to produce a standardized DR. The weights used were obtained from the maternal age distribution of pregnancies in England and Wales in 2011 and the maternal age specific probability of euploid, T13, T18 and T21.
Similarly, standardized FPRs were computed by obtaining the likelihoods in unaffected pregnancies and then applying these to each year of maternal age from 12 to 50 years to estimate the age specific FPRs. These were then weighted according to the maternal age distribution of euploid pregnancies in England and Wales in 2011.
5.2 Results
Performance of screening depends on maternal age, for the population overall, the DR of T18 using the quadruple test is estimated to be around 75% and the FPR is estimated to be around 0.1% using a 1 in 150 at term cut-off.
The modelled performance of screening using the quadruple test with a cut-off of 1 in 150 applied separately to probabilities for T21 and T18/T13 are given in Table 3. This gives the proportions of screen positives for T21 alone, T18/T13 alone, both T21 and T13/T18 and of any of these. Performance for T13 is relatively poor and the majority of T13 pregnancies that screen positive, screen positive for T21 not for T18/T13. Including T13 along with T18 in the quadruple test increases the modelled detection rate of T13 by less than 1%.
| Condition | +ve for T21 alone | +ve for T18/T13 alone | +ve for both | +ve for any |
|---|---|---|---|---|
| T21 | 75.6% | 0.1% | 4.5% | 80.2% |
| T18 | 1.6% | 62.1% | 10.6% | 74.3% |
| T13 | 21.6% | 0.9% | 8.0% | 30.5% |
| Euploid | 3.2% | 0.1% | 0.0% | 3.3% |
Table 3: Performance of screening (screen +ve proportions) for the quadruple test obtained using a term cut-off of 1 in 150 for the probability of T21 and T18/T13.
The poor performance of screening for T13 is a consequence of low prior probability for T13 and the distribution of markers in T13 pregnancies. The prior probabilities of T13, T18 and T21 for maternal ages 20, 21, 22, 23 and 24 are shown in Table 4. For T18, they are about one twelfth as high as those for T21. For T13 they are about one thirtieth of those for T21. This means that the markers must be strongly informative of T18 or T13 relative to T21 to make the posterior probability of T18 or T13 higher than that of T21.
The median MoM levels for T13, T18 and T21 are shown in Table 5. The median marker profile for T18 is very distinct from that of T21 and from euploid pregnancies. As consequence of this, T18 pregnancies are well distinguished from both T21 and euploid pregnancies in the probability calculations. The median profile for T13 is not that distinguishable from euploid pregnancies nor from T21 pregnancies. This, together with the low prior probability for T13 relative to T21, is why the majority of T13 pregnancies detected are detected based on the T21 probability rather than T18/T13 probability.
| Maternal age (years) | T21 | T18 | T13 |
|---|---|---|---|
| 20 | 1 in 1500 | 1 in 18000 | 1 in 43000 |
| 21 | 1 in 1300 | 1 in 16000 | 1 in 38000 |
| 22 | 1 in 910 | 1 in 11000 | 1 in 25000 |
| 23 | 1 in 380 | 1 in 5000 | 1 in 11000 |
| 24 | 1 in 110 | 1 in 1000 | 1 in 3000 |
Table 4: Maternal age specific prior probabilities
| Marker | T21 | T18 | T13 |
|---|---|---|---|
| AFP | 0.74 | 0.72 | – |
| uE3 | 0.70 | 0.47 | – |
| hCG | 2.05 | 0.36 | – |
| Inhibin | 2.18 | – | 1.61 |
Table 5: Median MoM values for second trimester MoM values. The symbol – indicates that there is no evidence that the medians differ from Euploid pregnancies.
6. Conclusion
It is possible to produce quadruple test probabilities for T21 and for T18/T13 so the quadruple test in the second trimester is aligned with the combined test in the first trimester. However, with the quadruple test markers T13 is not well distinguishable from euploid pregnancies nor from T21 pregnancies. This means that screening performance for T13 is relatively poor and that the majority of T13 pregnancies that are screened positive, screen positive for T21 but, not for T18/T13.
We conclude that the option of screening for T18 could be made available in the NHS FASP second trimester quadruple test but not screening for T18/T13.
7. Authors
Dave Wright, Statistician, Professional Clinical Advisor, NHS England
Nadia Permalloo, Quality and Improvement Lead, NHS England
Alan Wright, Statistician, Professional Clinical Advisor, NHS England
Nick Aldridge, Robin Glover, Jennifer Broughan, NCARDRS
Original paper January 2022, updated April 2023
-
Data source – NCARDRS data 2019/20. ↩
-
Bestwick JP, Huttly WJ, Wald NJ. Detection of trisomy 18 and trisomy 13 using first and second trimester Down’s syndrome screening markers. J Med Screen. 2013 Jun;20(2):57-65. doi: 10.1177/0969141313484904. Epub 2013 May 28. Erratum in: J Med Screen. 2015 Mar;22(1):52-4. PMID: 23761419. ↩

