Life Sciences Sector Deal 2, 2018
Updated 5 December 2018

Illustration of a molecule (credit: BlackJack3D/iStock - ID:682373850) [Credit: BlackJack3D/iStock]
Foreword
Last year the Life Sciences Sector Deal set out the first phase of implementing the Life Sciences Industrial Strategy through joint commitments between the government and the sector to invest in the United Kingdom’s life sciences landscape.
The agreements made in the first deal capitalised on the enormous opportunities for growth that the life sciences sector presents for the UK. We are seeing a continual flow of new investments across the health tech and pharmaceutical sectors since the deal was published, with this year’s second deal seeing more life sciences companies investing in the UK and creating high-quality, well-paid jobs. Life sciences is a sector that operates at the cutting-edge of technological developments.
Government investments in these emerging areas are being matched by greater contributions from the sector, and this is building on existing strengths spanning research and healthcare to create and grow new industries in the UK. It is right that NHS patients stand to benefit from these home-grown innovations. Data-driven technologies have the potential to transform the way the health system works and support faster and cheaper clinical research. We must ensure the system develops to encourage and spread these new technologies. Advanced therapies are making precision medicines, targeted to the individual patient, a reality here in the UK.
This second Life Sciences Sector Deal deepens our partnership with industry, universities and charities and demonstrates how the NHS is pivotal as a delivery partner. It contains significant action to address the Industrial Strategy Grand Challenges and our mission to transform the prevention, diagnosis and treatment of chronic diseases by 2030: it will support the creation of a cohort of healthy participants that will enable research into the hidden signs of disease and ways of diagnosing diseases early when interventions and treatments can be the most effective. The cohort will involve the public, the research community and industry with the results benefiting the whole health service – an example of the collective endeavours driving our modern Industrial Strategy.
We are taking action in the work underway from the first Sector Deal, and the new commitments set out in this deal, to achieve the vision of the UK as a top-tier hub for life sciences globally. The benefits will be felt by UK citizens through economic growth, good jobs and a strong and sustainable health service for future generations.
Rt Hon Greg Clark MP
Secretary of State for Business, Energy and Industrial Strategy
Rt Hon Matt Hancock MP
Secretary of State for Health and Social Care
Professor Sir John Bell GBE, FRS
Regius Professor of Medicine, The University of Oxford
Executive summary
The life sciences industry is one of the most important pillars of the UK economy, contributing over £70 billion a year and 240,000 jobs across the country[footnote 1]. In 2017, a wide coalition of industry and charity partners, led by Professor Sir John Bell, published a bold Life Sciences Industrial Strategy[footnote 2] that set a clear direction for the sector’s future growth.
The strategy was underpinned by the very significant competitive advantages the UK already possesses – an outstanding research base in world-class universities; significant capabilities in clinical and translational medicine in the NHS; longitudinal datasets within a national healthcare system; the largest biotech cluster outside the United States; a highly-productive and skilled workforce; and strong capabilities in emerging fields, such as digital health and artificial intelligence (AI).
Taking these strengths as the basis for future growth, the strategy set out how to maintain and build on them under fierce international competition, and to capitalise on emerging opportunities. It set a roadmap for the UK to create whole new industries over the next 2 decades in the fields of early disease detection and genomics; digital technologies and data analytics; and in advanced therapeutics.
The strategy also set out the areas where key developments could fix issues which have slowed down growth; such as NHS uptake of innovative technologies; the difficulty in scaling UK biotech companies to create large, globally-successful entities; and the need to drive up research and development funding to remain globally competitive.
The government’s response to this call from industry came with the first Life Sciences Sector Deal[footnote 3], a key part of the modern Industrial Strategy. The deal included close to £500 million of government support for major new research programmes and over £1 billion of new industry investment. It brought together partners from across the pharmaceutical and health tech sectors, charities, government agencies and the NHS to deliver its vision at pace. Strong progress is being made on implementation:
- An investment of £85 million in our already world-leading genomics assets at UK Biobank has attracted significant support for what will be the largest whole genome sequencing project ever undertaken. A £30 million vanguard study, the first phase of work, is already underway, sequencing the first 50,000 participants.
- In digital technologies and data analytics, we are laying down the building blocks to realise the full potential of the scale and diversity of NHS data, while maintaining public trust in how data is used and maximising the benefits of innovation for NHS patients. Our 5 new centres of excellence in digital pathology and radiology will provide more precise diagnosis, applying AI tools to digital images to detect abnormalities more quickly and accurately than humans. They will also help to reduce costs.
- We are delivering on our aim to make the UK a global hub for advanced therapies and building an impressive end-to-end national infrastructure through a £146 million commitment to medicines manufacturing. This includes: doubling capacity at the Cell and Gene Therapy Catapult Manufacturing Centre; 3 new advanced therapies treatment centres; and 2 new innovation centres for vaccines and medicines manufacturing.
- The government, industry, the NHS and its partners are delivering on their clear commitment to work together to improve the innovation and access environment in the UK, with strong progress made on implementing the Accelerated Access Review[footnote 4], supported by £86 million in government funding.
One year on, we are now going further still through this second Life Sciences Sector Deal with significant additional measures and innovative programmes to secure a global lead in the areas of greatest opportunity for the UK. Together with the first Sector Deal, these programmes are building an ecosystem that will help life sciences to thrive in the UK:
- A major new commitment, backed by up to £79 million of funding, to develop a first-of-its-kind, world-leading cohort of healthy participants that will attract significant global investment from industry and charities. The project will enable research into the hidden signs of disease and the development of tools to detect and diagnose diseases earlier. This will lead to a type of ‘predictive prevention’ which is crucial to ensure the NHS has a sustainable future.
- A world-first commitment to sequence 1 million whole genomes in the UK within the next 5 years including 500,000 through the NHS in addition to the 500,000 in the UK Biobank project. Our broader aspiration is to reach 5 million genomic analyses in the same time-frame, truly making the UK the home of genomic healthcare.
- An additional £50 million investment in our digital pathology and radiology programme as a first step towards making this a truly national asset to support early and improved diagnosis across the UK and deliver more efficient NHS services.
- £37.5 million funding and a plan for a network of regional Digital Innovation Hubs, providing expert clinical research data services, data analysis and sharing capabilities. This is part of a wider programme delivering on the government’s tech vision to reform the architecture of technology in the NHS and make it work better for patients, clinicians and researchers.
- A wide-ranging new policy package to support uptake and adoption of innovation in the NHS and ensure the UK continues to be a world leader in health tech. Through its long-term plan and the recent branded medicines pricing deal, the NHS will set out how it will be a crucial national partner, acting as both a real-world test-bed and a beneficiary of many of the innovations flowing from the life sciences industry.
Wider policy measures are reinforcing this approach, including:
- A suite of measures to make our clinical research environment faster, more efficient, streamlined and innovative, including the use of digital platforms.
- A commitment to innovative regulation that ensures the UK framework keeps pace with emerging technology developments, such as artificial intelligence, and supports their entry into the NHS.
- A new package of skills commitments between the government and industry partners to help deliver the priority skills the sector needs now and to make the most of future opportunities.
- Ongoing work to deliver on our commitment to increase public and private R&D spend, reaching 2.4% of GDP by 2027
- Measures to support the supply of capital for innovative firms, helping them to grow, through the government’s Patient Capital Review[footnote 5]
Industry partners have responded to this significant commitment with further investment:
- UCB, a global pharmaceutical company, is investing in 1 of their 2 major global R&D hubs in the UK. This will involve a purpose-built, state-of-the-art facility to enable cutting-edge R&D, early manufacturing and commercial operations, with planned investment of £150 million to 200 million in the facility and around £1 billion total investment over 5 years. The transition to the new facility will support around 650 high-value jobs across R&D, enabling further collaborations with UK universities, biotechs and medical research charities
- Over £200 million of new investment from a wide range of companies, including GW Pharmaceuticals, Roche, Celgene, IQVIA Ltd. and Oxford Biomedica
The strength of the partnership between the government, the NHS and the life sciences sector is making the UK a global standard-bearer for discovery research and advanced manufacturing. We are committed to continuing the hard work of implementation over the coming years because the prize – a globally-leading UK life sciences environment – will deliver huge benefits to the people of this country through a stronger economy and a stronger NHS.
Key commitments
Ideas
To be the world’s most innovative economy.
Establishing the Health Advanced Research Programme
Life Sciences Sector Deal 1:
The government committed to initial collaborations in genomics and digital diagnostics, with leading health charities exploring concepts and structures to shape the future of the Health Advanced Research Programme.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| * Wellcome Trust, the Bill & Melinda Gates Foundation and Cancer Research UK have come together with the government to define the concept of a Health Advanced Research Programme. | * The government is announcing a major new commitment to the ‘Accelerating detection of disease’ challenge, backed by up to £79 million from the Industrial Strategy Challenge Fund[footnote 6]. The challenge will build a world-first cohort of healthy participants to support research, early diagnosis, prevention and treatment across the major diseases. |
| * Wellcome Trust has committed to progressing very high-risk/high-reward pioneering science through a major £250 million commitment to their Leap Fund. | * Committing an additional £50 million investment in our digital pathology and radiology programme to help make this a truly national asset and deliver more efficient NHS services. |
| * Prof Sir John Bell will bring together leading health charities including Cancer Research UK, British Heart Foundation and Alzheimer’s Research UK on the government’s ‘Accelerating detection of disease’ challenge, which we also expect to attract significant further investment from the sector. | * Committing to sequence at least 1 million whole genomes within 5 years, including 500,000 whole genomes through the NHS. |
| * Genomics England will undertake development work on a new service to enable genomic volunteers to pay for a personalised report on their unique genetic makeup. With permission, the genetic data will be made available to researchers and scientists. |
Strengthening the UK environment for clinical research
Life Sciences Sector Deal 1:
Significant action has been taken over the last year to strengthen the environment for clinical trials. Sector collaborations between companies and academia are developing innovative clinical trials.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| The government’s comprehensive plan to improve our clinical research environment has unlocked new commitments from companies: | We will further improve the speed and efficiency of clinical trials by: |
| * Celgene provides support for studies and is making a new investment in excess of £7m, with an overall £38m investment. | * Establishing 5 centres for late phase commercial research. |
| * With National Institute for Health Research (NIHR) facilitation support, IQVIA Ltd. will invest £24m over 5 years into a fourth UK Prime Site for clinical trials across the North of England. | * Exploring opportunities to recognise and incentivise NHS Trusts and GP practices acting as participant identification centres. |
| * IQVIA Ltd. and Genomics England are investing £20m over 5 years to develop services that will enable more efficient drug research. | * Continuing to improve research set-up timelines. |
| * The Brain Tumour Charity will invest £2.8m in the Tessa Jowell BRAIN-MATRIX, a trial aimed at increasing opportunities for brain tumour patients to try non-standard treatments. | * Addressing challenges in NHS workforce resourcing to deliver commercial research. |
| We will consolidate our world-leading position in delivering novel and innovative trials by: | |
| * Promoting the UK’s expertise in designing and delivering innovative trials. | |
| * Enabling industry, including SMEs, and the wider research community to access advice to support innovative trial design. | |
| * Delivering a skills programme to embed expert understanding of how innovative studies can be run across the NHS. |
Raising the intensity of R&D in the UK
Life Sciences Sector Deal 1:
Last year’s deal highlighted the commitment to increase investment in R&D to 2.4% of GDP by 2027 and various company investments.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| We are seeing further commitments from companies demonstrating confidence in the UK’s science base: | We will build on action to increase the UK’s R&D spend to reach 2.4% of GDP by 2027 by: |
| * UCB will invest up to £150-200m in a state-of-the-art global R&D hub – a commitment which totals around £1bn over 5 years and will support 650 jobs. | * Working across UKRI and industry to develop a roadmap to achieve 2.4%. |
| * Roche will invest a further £30m in the UK including 100 new highly-skilled jobs by 2020. | * Announcing additional support at Budget 2018, including £1.6bn to strengthen the UK’s leadership in science and innovation. |
| * Celgene are investing over £22m in a 5-year drug discovery collaboration with Cancer Research UK. | * Setting the ambition to triple industry contract and collaborative R&D spend in the NHS to over £900m. |
| * Further oncology R&D investments include: £14m from Cancer Research UK into a London hub for cancer biotherapeutics research and treatment; £2.4m investment by Bristol-Myers Squibb into a project with the Francis Crick Institute and Cancer Research UK into AI analysis of lung cancer images. | |
| * An Electron Bio-Imaging Centre was opened by Diamond Light Source backed by a £15.6m grant from Wellcome Trust and UKRI, with £9m from ThermoFisher Scientific. | |
| * Eisai announced an extension to its UK investment in dementia research, a new 5-year collaboration with UCL. |
Business environment
To be the best place to start and grow a business.
Making the UK a global hub for advanced therapies manufacturing
Life Sciences Sector Deal 1:
The below measures build on £146 million support for medicines manufacturing from the Industrial Strategy Challenge Fund (ISCF), leveraging significant matched industry funding.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| * Investors have recognised the strength in UK advanced therapy biotech companies with Orchard Therapeutics Plc, Autolus Ltd. and Freeline Therapeutics Ltd. raising significant finance in the second half of 2018. | Capitalising on the opportunities presented by advanced therapies: |
| * Bellicum Pharma Ltd., a cell therapy company, has committed to its first European investment in the UK with £2m and 20 jobs initially. | * A significant portion of the £146m ISCF support for medicines manufacturing announced in the first sector deal has been targeted at advanced therapies. |
| * UK companies are scaling up cell and gene therapy manufacturing including Oxford BioMedica Plc (£19m) and Roslin Cell Therapies Ltd. (£4m) planning to invest. | * NHS Blood and Transplant are expanding their infrastructure to facilitate adoption of cell and gene therapies. |
| * Autolus Ltd. is planning to invest a further £50m to expand its UK presence, including a new global headquarters. |
Supporting growth of life sciences manufacturing
Life Sciences Sector Deal 1:
In addition to advanced therapies manufacturing, last year’s Sector Deal committed support to drive growth in high-value medicines manufacturing, including for 2 new innovation centres for Vaccines and Medicines Manufacturing.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| Industry are making new investments in life sciences manufacturing facilities and technologies: | The government is going further to build on progress seen in life sciences manufacturing since the sector deal: |
| * GW Pharmaceuticals has invested over £20m expanding its manufacturing facilities in Kent. | * £8m ISCF funding was announced as an initial step to facilitate the application of digitally-enabled technologies for medicines manufacturing. |
| *Eli Lilly and Company has committed £5m to research into more efficient medicines manufacture, in collaboration with Imperial College London and UCL. | * Up to £121m was announced for the Made Smarter Programme through ISCF wave 3 to support the transformation of manufacturing, including in life sciences. |
Improving UK environment for businesses to scale up
Life Sciences Sector Deal 1:
Last year’s deal outlined the government’s response to the Financing Growth in Innovative Firms[footnote 5] consultation, addressing scale-up issues with an action plan to release over £20 billion of patient capital investment over 10 years. We are now going further still through announcements made at Budget 2018.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| * Many of the UK’s largest defined contribution pension providers will work with the British Business Bank to explore pooled investment in patient capital. | * The government is going further to increase opportunities for pension funds to invest in the sector by addressing regulatory barriers and announcing plans to help them invest in growing UK businesses through the British Business Bank (BBB). |
| * The UK BioIndustry Association is conducting analysis of life sciences investment to inform a campaign to promote investment and growth opportunities. |
NHS innovation and collaboration
Life Sciences Sector Deal 1:
The government committed to implementing the Accelerated Access Review, including £86 million funding to support innovators and the NHS. The sector is delivering collaborative programmes in the NHS to transform services and improve care.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| Collaborations are demonstrating improvements to patient services: | We are now going further and committing to: |
| * The wound care industry is working with Academic Health Science Networks to improve patient outcomes and help local areas maximise budgets. Between them Smith and Nephew and Convatec are investing over £20m in UK R&D and academia. | * Build a stronger innovation ecosystem through a much enhanced and strengthened Accelerated Access Collaborative (AAC). |
| * Barts Health NHS Trust, Queen Mary University of London and other partners are working with the Department of Health and Social Care to develop a major new life sciences hub in Whitechapel. Total investment is expected to reach £500m. | * Improving patient access to more innovations – the AAC will be given greater flexibility over the number of products it can support, as long as they deliver good value. |
| * Improve the innovation infrastructure that underpins the AAC’s work through stronger testing infrastructure. | |
| * Tackle the challenge of spreading the best innovations across the system through a new health tech funding requirement. |
Innovative regulation
Life Sciences Sector Deal 1:
Last year’s deal included a suite of actions to support the UK environment for life sciences businesses. An effective regulatory environment that works for innovative, emerging new technologies is key to this.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| The sector will work with the Medicines and Healthcare Products Regulatory Agency through its industry liaison groups to provide input and expertise as the agency develops its approaches to ensure that the regulatory framework delivers a strong business environment. | The Medicines and Healthcare Products Regulatory Agency (MHRA) has set out how it will be the most forward-thinking regulator, essential for a strong business environment for life sciences, by: |
| * Supporting advanced therapies manufacturing by developing a framework for point-of-care manufacture. | |
| * Leading the way on precision medicine by developing a clear UK regulatory pathway for genomic medicines and tests. | |
| * Promoting patient access and safety. |
Infrastructure
A major upgrade to the UK’s infrastructure.
Life Sciences Sector Deal 1:
The government committed to support the development of measures to improve the UK’s health data infrastructure, working with NHS England, NHS Digital and Health Data Research UK.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| Industry is helping to pioneer the use of digitally-enabled research and digital technologies: | We are making the UK the home of data-driven life sciences research and innovation, improving outcomes for patients and the NHS by: |
| * The ORION-4 trial is a partnership between the University of Oxford’s Clinical Trial Service Unit, its Epidemiological Studies Unit and The Medicines Company on a new cholesterol-lowering investigational drug given just once or twice a year using digitally-enabled tools to lower costs, and identify sites and participants more easily. | * Ensuring secure and appropriate use of patient data. |
| * A group of innovators will be invited to validate their algorithms on synthetic datasets created as part of MHRA’s Regulator Pioneer Fund project. This pilot ultimately aims to prove the concept and act as a regulatory sandbox to help with product validation. | * Creating the right framework for commercial agreements involving data. |
| * Improving the UK’s health data infrastructure, including through investments to improve NHS Digital’s core services; and to develop the Local Health and Care Record Exemplars and the Digital Innovation Hubs programmes. | |
| * Supporting and expanding digitally-enabled clinical research. | |
| * Creating a regulatory framework fit for the future and able to keep pace with technological developments, such as AI. The MHRA has secured £740,000 from the Regulator’s Pioneer Fund to work with NHS Digital on developing a pilot in order to test and validate algorithms and other AI used in medical devices. |
Places
To have prosperous communities throughout the United Kingdom.
Life Sciences Sector Deal 1:
The government committed to implement a regional approach to the Sector Deal, working closely with devolved administrations and cluster organisations representing the sector.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| The sector is proactively identifying growing areas of opportunity for life sciences in the UK regions and devolved administrations: | We are taking action to help areas with clear life sciences strengths to grow: |
| * Bruntwood in partnership with Legal & General Capital are investing £360m of capital, property and intellectual assets to create the UK’s largest portfolio of science and technology assets in the Northern Powerhouse and Midlands Engine initially. | * The government is renewing our offer of Life Sciences Opportunity Zone status to help areas raise their profile at an international level. |
| * New projects and partnerships are being developed across the devolved administrations and English regions, generating significant investment throughout the UK. | * The Department of International Trade, with the Office for Life Sciences and leading cluster organisations will work together to make the life sciences landscape easier for investors to navigate and support growth. |
| * Budget 2018 announced £20m to further develop the plan for the critical central section of East-West rail between Oxford and Cambridge. |
People
To generate good jobs and greater earning power for all.
Life Sciences Sector Deal 1:
The government and sector committed to work together to monitor the impact of the apprenticeship levy and to support standards brought forward by the sector.
Life Sciences Sector Deal 2:
| Sector action | Government action |
|---|---|
| The sector is developing new joint programmes targeted at key skills needs: | We are working to increase uptake of life sciences apprenticeships: |
| * Encouraging young people to study STEM subjects, eg The Association of the British Pharmaceutical Industry (ABPI) is supporting the British Science Association’s work to inspire young people about STEM through a competition around the Industrial Strategy Grand Challenges. | * The government is taking action on the apprenticeship levy to facilitate greater flexibility and improve uptake, particularly for SMEs. |
| * The Science Industry Partnership (SIP), with key partners, including ABPI and the BioIndustry Association will lead and deliver a Life Sciences 2030 Skills Strategy, funded by £100,000 from SIP, with further funding from trade association partners and the government. | * We are also committing to explore the potential for a pilot programme with UK Research and Innovation (UKRI), the Department for Education and the Education and Skills Funding Agency to better enable SMEs in the sector to take on apprentices. |
| * SIP is delivering on commitments to roll out regional approaches on skills, including apprenticeships uptake by building on the success of SIP Cambridge with a commitment to establish SIP Liverpool. | |
| The government is taking a range of further measures to develop the skills the sector needs, including: | |
| * Increasing uptake of science, technology, engineering and maths (STEM), through a new external working group, led by the Department for Education. | |
| * New UKRI programmes that are helping to deliver on opportunities that emerge through joint working between life sciences and other disciplines. |
Ideas

Illustration of a DNA strand (credit: iLexx/iStock - ID:485038074) [Credit: iLexx/iStock]
Establishing the Health Advanced Research Programme (HARP)
The Life Sciences Industrial Strategy set out a bold vision for a pioneering Health Advanced Research Programme (HARP) that would put the UK at the forefront of addressing the unprecedented challenges facing healthcare systems globally. It proposed that industry, charities and the NHS take on far-sighted, life sciences projects, underpinned by novel technology and higher-risk science. In the first Life Sciences Sector Deal, the government committed to working with partners to explore how this vision could be achieved.
In the last year, leading charities, including Wellcome Trust, the Bill & Melinda Gates Foundation and Cancer Research UK have come together with the government to determine how to make a success of the enormous opportunity HARP presents. This work identified 4 core categories of science and research opportunities that, taken together, create a powerful portfolio that will help the UK lead the way in tackling major global healthcare challenges.
Backed by £210 million of Industrial Strategy Challenge Funding (ISCF) from UK Research and Innovation (UKRI), the first set of HARP projects are now becoming a reality.
Category 1: Investing in long-term assets to sustain health research
In the first Life Sciences Sector Deal, we committed to a ground-breaking project to build on our world-leading genomics assets and ensure that the UK remains globally competitive in this area. The UK already has the largest, highest-quality whole genome database in the world through the 100,000 Genomes Project, which reached its target in December, but the first Sector Deal recognised that we cannot afford to stand still in the face of global competition.
While we are still at the early stages of using genomics to treat rare and hard-to-treat conditions, the strategic direction is that over the next 10 years the volume of genomic data combined with insights derived from clinical data and experiential data – including from wearables and Internet of Things devices – means genomics has the potential to become a key pillar of preventative healthcare, enabling much more precise advice and treatments.
In the space of the last year, the government has allocated £85 million of ISCF funding to kick start the whole genome sequencing of all 500,000 UK Biobank participants.
This will be the largest whole genome sequencing project ever undertaken and should be completed in 2 years. A £30 million vanguard study, representing the first phase of work, is already underway with the sequencing of the first 50,000 participants having begun in August 2018. And this project has already generated significant interest and potential new investment to the UK: several companies and charitable organisations are forming a partnership to work with the government on the project, demonstrating that the UK remains a leading destination for investment in cutting-edge genomic research. The government is continuing to work with Genomics England and other partners to progress the cancer genome sequencing announced in last year’s deal.
The government is now going further and faster in its commitment to drive advancements in personalised, targeted healthcare for the benefit of patients. In October 2018, the Health and Social Care Secretary announced an ambition to become the global leader in genomic care through:
- Launching the most ambitious genomics programme in the world to sequence at least 1 million whole genomes within 5 years, including 500,000 whole genomes through the NHS in addition to the 500,000 in the UK Biobank project. Our broader aspiration is to reach 5 million genomic analyses in the same time frame, truly making the UK the home to genomic healthcare.
- Making the NHS the first national healthcare system in the world to offer whole genome sequencing as part of routine care for NHS patients. During 2019, the new National Genomic Medicine Service will begin to offer whole genome sequencing to seriously ill children likely to have a rare genetic disorder and children with cancer, in addition to adults suffering from certain rare conditions or specific cancers.
In addition, Genomics England will undertake detailed development work on a new service to enable genomic volunteers to pay for a personalised report on their unique genetic makeup. As part of this and with the permission of these volunteers, the genetic data will be made available to researchers and scientists working on tackling some of our country’s greatest health challenges. Genomics England will work with NHS England and public and patient groups to lead the development of the service.
This joint commitment from the government and our industry and charity partners to building unique, long-term genomics assets will help attract further investment from the biopharmaceutical industry seeking to use large-scale genomic data to validate targets and produce more successful medicines. It will also enable the development of new and emerging UK-based technology companies, for example in DNA sequencing and annotation.
Category 2: Development programmes applying cutting-edge technology and science to current problems
The Life Sciences Industrial Strategy highlighted the important role artificial intelligence can play in healthcare –helping to reduce costs and provide more precise, earlier diagnosis – for example, transforming NHS services such as radiology and pathology by detecting abnormalities in digitised scans and tissue samples more quickly and accurately than humans.
In the first Life Sciences Sector Deal, the government committed to work with companies to shape a programme of NHS and industry collaboration, backed by Industrial Strategy Challenge Funding, that will ultimately develop and demonstrate new solutions at scale. Since then, the government has completed a competition to establish a network of 5 centres of excellence in digital pathology and radiology with AI, supported by £50 million from the Industrial Strategy Challenge Fund, to demonstrate how this can work in practice in the NHS. Major players including Philips, Roche Diagnostics, Canon, Siemens Healthineers, GE Healthcare and Leica are key partners with the NHS and academia in this ground-breaking programme, which has leveraged over £33 million in investments from industry and other sources and will put the UK at the forefront of leadership in digital pathology and radiology using AI.
The centres will work with innovative SMEs to identify, develop and learn new processes, practices, knowledge and skills, and to develop new products and services for the benefit of the NHS and patients that also have global commercial applications. Centres will work closely within their regional NHS partnerships, as well as national infrastructure, to develop and implement interoperability standards and to commit to the adoption of national standards as these are defined.
Coupled with other significant government investments in genomics, the image analysis from the centres will revolutionise understanding of the development of complex diseases, enabling the development of new tools for early diagnosis and new treatments targeted at specific diseases. The programme is an early deliverable under the Prime Minister’s Early Diagnosis and AI mission, demonstrating the opportunity for digitising care and using data with AI to enable scientific research and develop AI-based diagnostic tools.
In this second Life Sciences Sector Deal, the government is now going further and announcing additional investment of up to £50 million in our digital pathology and radiology programme as a first step towards making this a truly national asset to support early and improved diagnosis across the UK, and deliver more efficient NHS services. This will accelerate the speed at which the NHS and patients are able to realise the benefits from our investment and enable these benefits to be shared by everyone.
Category 3: Visionary programmes looking 20 years ahead to revolutionise healthcare
The Life Sciences Industrial Strategy highlighted an opportunity for the UK to lead the way globally in changing the healthcare paradigm so that we are proactive in identifying, preventing and treating disease early, rather than reactive in seeking to manage late-stage illness or merely prolong life. Since the Sector Deal last year, the Prime Minister has announced a mission to use data, AI and innovation to transform the prevention, early diagnosis and treatment of chronic diseases such as cancer, diabetes, heart disease and dementia by 2030, whilst the Health and Social Care Secretary has set out his vision for an NHS which spends much more of its resources on keeping people well and healthy for longer.
The scale of opportunity is enormous: success would mean saving many more lives, reducing NHS costs, attracting investment to the UK from global pharmaceutical and health tech companies, and helping new UK industries, for example, in data analytics and diagnostics, to grow. It would also empower all UK citizens to understand their individual risk of developing diseases as never before, and take steps to remain healthy for longer. In the UK, we are spending £97 billion a year on treating diseases, and only £8 billion on preventing them[footnote 7]. Cancer Research UK estimates that by 2033, if the number of late stage diagnoses were reduced by 50% just across bowel, ovary, prostate and lung cancer, each year over 55,000 more people would be diagnosed at an early stage rather than a late stage. This could result in over 22,000 fewer deaths within 5 years of diagnosis, per year[footnote 8].
In this second Life Sciences Sector Deal, the government is announcing a major new commitment of up to £79 million R&D funding[footnote 9] in the Accelerating Detection of Disease challenge under the Industrial Strategy Challenge Fund Wave 3, that is expected to leverage significant industry and charity matched funding from the life sciences sector. This will help deliver the Early Diagnosis Mission, announced by the Prime Minister in May 2018 – a key part of the Industrial Strategy’s AI and Data Grand Challenge – as well as delivering on a major recommendation in the Life Sciences Industrial Strategy.
Professor Sir John Bell will bring together UKRI, Cancer Research UK, the British Heart Foundation, Alzheimer’s Research UK and other leading health charities, the NHS and industry to build a first-of-its-kind, world-leading cohort of 1 million healthy participants initially, aiming to grow to 5 million. The project will support research, early diagnosis, prevention and treatment across the major diseases, including cancer, dementia and heart disease. This will be a ground-breaking national health programme that will develop new diagnostic tests through applying leading-edge AI and other cutting-edge technologies. It will attract additional global investment from the sector.
Case study: Leading the way in the early diagnosis of cancer
Oncimmune is a leading UK cancer diagnostic technology that has pioneered the development of a blood test that has the potential to transform the detection and diagnosis of cancer through the measurement of autoantibodies. The technology is being used as part of the Early Cancer detection test – Lung cancer Scotland (ECLS) study, which is believed to be the largest randomised trial for the early detection of lung cancer using a blood-based biomarker ever conducted.
The ECLS study is specifically targeted at a population with a lower risk of developing lung cancer, 0.5% compared to 2% or even 3.5% in other screening studies, successfully recruiting over 12,000 participants.
By utilising a simple blood test run on inexpensive standard laboratory equipment, the technology is frictionless, resulting in an unprecedented rate of recruitment compared to similar screening studies. The test was able to identify a small population for follow-up when compared to other screening programmes, significantly reducing the risks of false positives associated with large-scale screening trials.
Interim results have demonstrated that Oncimmune’s technology has the potential to save lives and avert costs to the NHS by detecting many more early-stage cancers (75% earlystage versus 20% today). By using a blood test, the screening approach is more acceptable to participants and has fewer implications for NHS imaging capacity.
Category 4: Very high-risk/high-reward pioneering science to create new technologies and breakthroughs
The Life Sciences Industrial Strategy was clear that delivery of the Health Advanced Research Programme’s (HARP) ambitions would require a coalition of partners and that charities would play a leading role.
This is particularly true of high-risk, high-ambition programmes that fall outside the remit of conventional life sciences funding because of the high level of risk involved.
Wellcome Trust has decided to play a significant role in filling this gap through a major new £250 million commitment to their far-reaching Leap Fund programme. This new, not-for-profit fund will place big bets on ambitious research programmes with the potential to fundamentally change science or transform health over a 5-to-10 year horizon. The fund will support researchers, engineers, innovators and technologists from around the world to pursue bold ideas at scale and speed, drawing inspiration from the technology and venture capital industries.
Strengthening the UK environment for clinical research
The UK’s expertise in clinical research is world-leading.
We continue to punch above our weight in many areas and rank second globally for commercial Phase 1 studies[footnote 10]. In 2017 to 2018, the National Institute for Health Research (NIHR) Clinical Research Network achieved record levels of recruitment with over 725,000 people taking part in clinical research studies, of which over 50,000 participants were recruited to studies sponsored by the life sciences industry, including 24 global-first patients. 99% of NHS Trusts were actively engaged in clinical research[footnote 11].
Responding to Life Sciences Industrial Strategy recommendations, the first Sector Deal committed to further improvements in our clinical research environment. Since then we have:
Made significant progress in delivery of NHS England and the NIHR’s commitments to 12 Actions to support and apply research in the NHS[footnote 12] by
- Awarding more than £950 million NIHR investment in research infrastructure in the NHS to translate findings from basic science to patient and healthcare benefit.
- Making it significantly easier to set up a study by implementing a new national model to manage excess treatment costs in non-commercial research.
- Simplifying processes for NHS research through a standard costing methodology, a model site agreement in the NHS standard contract and beginning to implement a single contract review to increase transparency and reduce variation.
- Removing the 70 day benchmark for clinical trials in favour of the publication of accurate performance data using a standard national framework[footnote 13].
- Increasing the uptake of the Clinical Practice Research Datalink in GP practices in England by almost 30% since the end of 2017.
- The Health Research Authority (HRA) and the Medicines and Healthcare Products Regulatory Agency (MHRA) piloting improvements to the approvals process - by offering a combined approach, researchers are receiving better quality and more efficient approvals in parallel rather than in sequence – which we are now rolling out more widely[footnote 14].
The life sciences sector is also delivering on commitments made in the first Sector Deal demonstrating that the UK is already a great place to do innovative clinical trials.
- Using anonymised electronic health records to improve efficient trial designs for mood disorders, the University of Oxford and Janss Pharmaceutica NV concluded that the NIHR-supported Clinical Record Interactive Search (CRIS) database can support effective identification of trial candidates. Wider application of this approach is being considered.
- The Medicines Company has begun the 2 projects announced in the first Sector Deal – the first with the University of Oxford to perform a large multinational cardiovascular disease clinical trial (see the ORION-4 case study). The second, with The Greater Manchester Health and Social Care Partnership, to improve the understanding, management and economics of cardiovascular disease has begun large-scale big data analysis to model the risk of heart disease using a medication to reduce certain risk factors.
- Wellcome Trust and the Bill & Melinda Gates Foundation are progressing with their aim of delivering more efficient and fit-for-purpose clinical trials for non-product registration trials. They are taking a global lead through an inclusive process in developing an alternative international equivalent to the good clinical practice guidelines, delivering on a recommendation in the Life Sciences Industrial Strategy. The African Academy of Sciences has recently become a partner of this joint initiative.
However, we can do more to improve our clinical research environment for both pharmaceutical and health tech companies. We will continue to improve speed and efficiency by:
- Establishing 5 purpose designed centres dedicated to late-phase commercial research in 2019 to 2020. Identified through NIHR Clinical Research Network (CRN) competition, the centres will offer rapid set-up of late phase commercial research, standardised contracting and delivery approaches where appropriate, and dedicated facilities and staff. They will increase the NHS’s capacity to deliver research, enabling significant growth and opportunities for patients to benefit from early access to innovation.
- Identifying opportunities to recognise and incentivise NHS Trusts and GP practices acting as participant identification centres. The NIHR CRN will explore mechanisms to ensure patients are offered the opportunity to participate in research of relevance to them, whether or not it is being delivered by their regular healthcare provider, making recommendations to the Department of Health and Social Care by spring 2019.
- Continuing to improve research set-up timelines by achieving HRA approval within timelines specified in the EU Clinical Trials Regulation; increasing the transparency of performance data through common use of the HRA/NIHR research study minimum dataset across NHS organisations, and providing industry with access to this data in a searchable format to inform site selection and intelligence-driven performance improvement.
- Addressing challenges in NHS workforce resourcing required to deliver commercial contract research. Working closely with industry and NHS Trusts, the NIHR CRN will investigate the workforce resource challenges in commercial contract research, and develop recommendations to government for innovative approaches to tackle them by summer 2019.
We also believe the UK can consolidate its world-leading position in delivering novel and innovative trials, making delivery of these studies ‘business as usual’ across the system. The Life Sciences Industrial Strategy proposed a strategic goal to grow the proportion of clinical trials with novel methodology over the next 5 years. Following on from excellent work by the Clinical Research Working Group, a partnership between industry and government, to identify innovation in trial design, we pledge to turn this vision into a reality by:
- Identifying and promoting the UK’s world-leading expertise in designing and delivering innovative trials, including opportunities for industry collaboration. We will work with NIHR early translational research infrastructure (NIHR Biomedical Research Centres, Clinical Research Facilities, and Experimental Cancer Medicine Centres) to make available details of their expertise in delivering innovative trials, making it easier for industry and other researchers to identify opportunities for collaboration. The NIHR CRN will also establish which NHS providers are able to deliver innovative trial designs to support faster site selection.
- Enabling industry, including SMEs, and the wider research community to access advice to support innovative trial design. The MHRA’s early engagement offer includes its Innovation Office where exploratory discussions can take place and a scientific advice service where experts can produce written guidance on a specific question relating to a product or study. This can be particularly helpful where trial aspects challenge the current regulatory framework. The MHRA will engage with industry to understand how it can further develop this offer by the end of 2019.
- Taking a range of measures to maximise the value of the UK’s unique data assets in clinical research, set out in full in the Infrastructure chapter.
- Delivering a skills programme to embed expert understanding of how innovative studies can be run across the NHS. The NIHR Clinical Research Network will deliver e-learning courses, designed to increase awareness and understanding of innovative trial designs, supplemented with targeted learning for specific groups.
- The government will explore with NHS England how they can publish an action plan on ways to optimise the use of real-world evidence.
Our comprehensive plan this year has helped to secure new commitments from the sector who recognise the UK as a leading place to invest and to undertake innovative trials:
- Celgene provides support for studies initiated by the researcher in areas including haematology, oncology and immunology. They are making a new investment in excess of £7 million and a total investment of £38 million. It will result in 12,500 UK patients receiving the best standard of care and novel treatments, free of charge to the NHS.
- With NIHR facilitation support, IQVIA Ltd. (formerly IMS Health & Quintiles) is committed to opening a fourth UK Prime Site to focus on the delivery of life-changing clinical research across the North of England. In addition, IQVIA Ltd. will invest up to £24 million over 5 years with the ambition of employing over 50 additional highly-skilled staff to drive additional trials across the UK. The Northern Prime Site will include NHS research-ready hospitals across the Greater Manchester, Leeds and Sheffield region, embracing a data-enabled approach to the design and delivery of hundreds of additional clinical trials and real world evidence studies to the benefit of thousands of NHS patients.
- IQVIA Ltd. and Genomics England have announced a new partnership, investing £20 million over 5 years to develop services to allow authorised researchers to run studies enabling faster and more efficient drug research, and to develop more robust evidence to support treatment value and greater access to personalised medicines for NHS patients.
- In May 2018, the Prime Minister announced £40 million over the next 5 years as part of the Tessa Jowell Brain Cancer Mission, doubling funding for brain tumour research. This will be invested through the NIHR to support a wide range of research and the mission’s running costs. The Brain Tumour Charity has now announced £2.8 million for the Tessa Jowell BRAIN-MATRIX trial, aimed at radically increasing opportunities for brain tumour patients to try nonstandard treatments. The NIHR will support the recruitment of patients, and start-up and delivery of the trial via the UK-wide Experimental Cancer Medicine Centres (ECMCs) and the Clinical Research Network. NHS England has also commenced a national neurosciences transformation project, a strand of which will focus on improving the pathway of care for neuro-oncology patients, building on existing work offering neuro-oncology patients access to dedicated outpatient clinics and pre-operative consultations with multidisciplinary teams. Health Education England is committed to ensuring the UK has leading neuro-oncologists to provide outstanding care and deliver ground-breaking research, so it is exploring further education and training opportunities such as credentialing in this area.
Raising the intensity of R&D in the UK
The UK has a world-leading science base, with 4 of the top 10 globally-ranked universities and with UK-based scientists winning more than 80 Nobel Prizes in chemistry, physics, and medicine - most recently winning chemistry prizes in both 2017 and 2018.
The Life Sciences Industrial Strategy emphasised the importance of building on this and continuing to increase R&D spend. In the Industrial Strategy white paper[footnote 15] and the first Life Sciences Sector Deal the government committed last year to work with industry to increase combined public and private expenditure on R&D to 2.4% of GDP by 2027, increasing to 3% over the longer term. In the last year UK Research and Innovation (UKRI) has been formally established to work in partnership with academia, research organisations, industry, charities, and the government to create the best possible research and innovation environment.
Building on this, Budget 2018 announced significant additional support for cutting edge science and technologies, including £1.6 billion to strengthen the UK’s global leadership in science and innovation. BEIS is working across industry and with UKRI to develop a roadmap that sets out the steps needed to reach the 2.4% ambition. A new ambition to triple industry contract and collaborative R&D spend in the NHS to over £900 million will provide a significant contribution to the overall 2.4% ambition.
Latest figures for 2017 show that the total spend on UK R&D by UK businesses was £23.7 billion. Of this, life sciences continues to be the largest contributor at 18% of the UK total, spending over £4 billion in R&D[footnote 16] and making excellent progress against significant commitments in the first Sector Deal:
- MSD announced last year that it would locate its new Discovery Centre in London. Since then, Dr Fiona Marshall was appointed as VP Head of Discovery Research at MSD UK and the plan is for 19 scientists to be in place by the end of the year, increasing to 150 new roles once the Centre is complete. The team of scientists will be studying cell homeostasis in the context of ageing that can lead to disease. The aim will be to identify mechanisms that can be targeted for new drug development for a range of diseases such as Motor Neurone disease, Parkinson’s disease and Alzheimer’s disease.
- In September 2018, Novo Nordisk opened its £115 million Oxford research centre, employing up to 100 scientists aiming to discover innovative treatments for Type 2 diabetes and cardiometabolic diseases.
As part of this second Sector Deal, we are seeing further new commitments from industry, reinforcing confidence in the UK’s R&D strengths and excellent science base:
The world-class research environment has led global pharmaceutical company UCB to invest in 1 of their 2 major global R&D hubs in the UK[footnote 17]. This will involve a purpose-built state-of-the-art facility to enable cutting-edge R&D, early manufacturing and commercial operations, with planned investment of £150 million to £200 million in the facility and around £1 billion total investment over 5 years. The transition to the new facility will support around 650 high-value jobs across R&D, enabling further collaborations with UK universities, biotechs and medical research charities, and build on UCB’s highly successful endeavours in researching and developing medicines in the UK to transform the lives of people living with severe diseases globally.
Roche will invest an additional £30 million in the UK, including £20 million over 3 years into a precision cancer research partnership with The Christie in Manchester. They will use cutting-edge foundation medicine genomic technology alongside big data to accelerate the next generation of digital clinical trials for rare cancers, making the UK a leading global hub for rare cancer trials and potentially benefiting nearly 5,000 patients annually. Roche will also invest £10 million into its Welwyn Garden City site in 2019 to 2020 while creating approximately 100 new highly skilled jobs.
Emphasising the strength of the UK cancer research ecosystem Celgene are investing over £22 million in a 5 year drug-discovery collaboration with Cancer Research UK to discover, develop and commercialise new anticancer treatments. This is one of many oncology R&D investments including: £14 million from Cancer Research UK to turn London into a world-leading hub for cancer biotherapeutics research and treatment; and a £2.4 million investment by Bristol-Myers Squibb to collaborate in a 1-year project, Rubicon, with the Francis Crick Institute and Cancer Research UK to assess the feasibility of using AI to analyse images of lung cancer study samples.
In September 2018, the Electron Bio-Imaging Centre (eBIC) in Harwell was opened by Diamond Light Source following the award of a £15.6 million grant from the Wellcome Trust and UKRI, with £9 million from ThermoFisher Scientific. Wellcome Trust subsequently invested an additional £9 million to significantly expand the facility. The facility is the first high-end cryo-electron microscopy (cryo-EM) facility worldwide to be embedded in a synchrotron and will firmly place the UK as a global leader in providing large-scale industrial access to cryo-EM for drug discovery and structural biology.
In May 2018, Eisai announced an extension to its investment in dementia research within the UK, a new 5-year collaboration with UCL, recognising the high standard of UK science and its universities.
In September 2018, KalVista Pharmaceuticals opened the anchor facility at the Porton Down Science Park, enabled by £5 million funding from 2 earlier Biomedical Catalyst grants. The facility will house its UK drug discovery and development group and will focus on development of new medicines for diseases with significant unmet need.
Business environment
Making the UK a global hub for advanced therapies manufacturing.

Pipette (credit: Randox Laboratories Ltd.) [Credit: Randox Laboratories Ltd.]
The first Life Sciences Sector Deal set out the government’s ambition to make the UK a global hub for advanced therapies manufacturing, responding to Life Sciences Industrial Strategy analysis that highlighted the emerging opportunities for the UK economy from new technologies such as cell and gene therapies. This ambition has been supported by a further £70.6 million of government funding for the Cell and Gene Therapy Catapult (CGTC) to build on our impressive existing national infrastructure and allow the UK to offer end-to-end capability in cell and gene therapies, from research to manufacturing, to clinical use and export, with the transformative benefits of these medicines being felt by NHS patients.
The UK’s capability in this field means we are securing highly-skilled jobs and accessing a global market estimated[footnote 18] to be worth between £9-14 billion per year by 2025. The UK is home to the highest number of cell and gene therapy commercial developers (64) in Europe, as well as the most cell and gene therapy clinical trials[footnote 19]. A cell and gene therapy cluster has formed in Stevenage, supported by the Stevenage Bioscience Catalyst and the Cell and Gene Therapy Catapult Manufacturing Centre: the Campus hosts 14 companies in this industry employing more than 350 people, that between them have raised over £680 million from commercial investors[footnote 20].
A significant portion of the £146 million Industrial Strategy Challenge Funding awarded for medicines manufacturing in the first Sector Deal has been directly targeted at advanced therapies:
- £12 million to double capacity at the Cell and Gene Therapy Catapult Manufacturing Centre. The CGTC opened its Manufacturing Centre in April 2018 to help cell and gene therapy developers build manufacturing capability, backed by £60 million of government investment. In September, the MHRA granted the Centre a Good Manufacturing Practice (GMP) licence, cementing its role as one of the world’s leading facilities for the development and production of cell and gene therapies. Companies are recognising the facility’s value and reaping the benefits. In 2018 2 further collaborators, Freeline Therapeutics and Adaptimmune, signed an agreement with the Catapult and discussions are already taking place for use of new capacity which is under construction at the facility.
- £5.5 million of capital support announced in the first Sector Deal has enabled leading UK viral vector manufacturers to expand their capacity:
- Cobra Biologics, an international contract development and manufacturing organisation in biologics and pharmaceuticals, received £2.5 million to support their £8 million investment in expanding their Keele site to enable production of commercial GMP quality viral vectors for advanced therapies, adding 25 new jobs onsite.
- £3 million of support enabled Oxford Biomedica to invest £19 million in a new manufacturing site which will enable them to target 25% to 30% of the global lentiviral vector bioprocessing market and create 100 new highly-skilled jobs.
- £21 million has been awarded to create 3 Advanced Therapy Treatment Centres (ATTCs). The ATTCs were set up in March 2018 to develop easily run solutions for the adoption of cell and gene therapies that can be rolled out across the NHS. The centres have attracted £5.7 million in co-funding from industry and involve over 20 different companies, in addition to NHS organisations, universities and national blood services.
- Over £12 million of the ISCF funding allocated for medicines manufacturing collaborative R&D projects has gone to advanced therapy projects, de-risking investment in innovation and attracting over £6 million of industry co-funding.
In addition to investments supported by Innovate UK, NHS Blood and Transplant (NHSBT) are expanding their human and capital infrastructure to facilitate the adoption of advanced cell and gene therapies. A new £16 million centre at Barnsley will include clean rooms to increase NHSBT’s capacity for the manufacture of advanced cell therapies, creating 12 highly-skilled new jobs. Further expansion of the Filton facility is proposed and will double NHSBT’s capacity for plasmid, viral vector and protein manufacture, creating 10 new specialist jobs.
These strong signals of UK commitment are generating significant industry activity:
- Investors have recognised the strength in UK-grown advanced therapy biotechs: Orchard Therapeutics raised $150 million (£117 million) from investors in Series C fundraising in August 2018 and $225 million (£175 million) in its initial public offering (IPO) in November 2018, to help it develop clinical programmes to treat rare inherited diseases. Autolus raised over £120 million through IPO in June 2018, while Freeline Therapeutics raised over £88 million of new capital in a Series B financing round.
- The UK’s ecosystem encouraged Bellicum, a clinical-stage cell therapy company, to locate its first European investment in the UK, initially committing £2 million and 20 jobs.
- UK companies are scaling up cell and gene therapy manufacturing capabilities: in addition to Oxford BioMedica’s £19 million investment in a new manufacturing site, we are able to announce that both Autolus and Roslin Cell Therapies Ltd. are investing in new manufacturing sites. RoslinCT, a cell and gene therapy contract development and manufacturing organisation is planning to invest a projected £4 million in the first phase of a new manufacturing facility in Edinburgh. This is expected to bring up to 50 new jobs and will provide commercial stage manufacturing services to advanced therapy developers.
Case study
UK-based company Autolus, spunout from University College London in 2014, is developing potentially life-changing T-cell cancer therapies. Initially supported by UK venture capital from Syncona, Woodford and Arix, and grants awarded by Innovate UK, Autolus recently raised over £120 million in a successful NASDAQ IPO. Autolus now employs more than 180 people. Building on its existing presence in London and as the first partner of the Cell and Gene Therapy Catapult Manufacturing Centre in Stevenage, Autolus is planning further investments of approximately £50 million in the UK. Over the next 3 years they intend to bring a new global launch manufacturing facility on-stream and expand their global headquarters and research laboratories, generating 100 high-value UK jobs.
Supporting the growth of life sciences manufacturing
The government has also supported a variety of projects that will drive up productivity in high-value medicines manufacturing more generally, in particular 2 pioneering new UK centres:
- The Medicines Manufacturing Innovation Centre, Strathclyde. The MMIC will help the UK lead globally in the development of process technologies for small molecule pharmaceutical and fine chemical manufacturing. The £56 million centre is supported by £13 million from the ISCF, as well as funding from Scottish Enterprise (£15 million), AstraZeneca (£7 million) and GSK (£7 million). It is expected to lead to £80 million in R&D investment by 2028, creating 80 direct jobs by 2023 with more indirectly.
- The Vaccines Manufacturing Innovation Centre, Oxford. The centre will help create new, cost effective ways of developing and manufacturing vaccines, building our resilience in the face of pandemic threats. Led by the Jenner Institute at the University of Oxford, Imperial College London and the London School of Hygiene & Tropical Medicine, the new centre has been awarded £66 million from the Industrial Strategy Challenge Fund. Additional funding of £10 million will come from commercial and other partners, including Janssen Vaccines & Prevention B.V. and MSD, and will be supported by bioprocessing expertise and training from GE Healthcare.
Government is continuing to go further to build on this strong progress:
- £8 million was announced in September 2018 as an initial step to facilitate the application of digitally-enabled methods or technologies to improve medicines manufacturing.
- Up to £121 million for the Made Smarter Programme through ISCF wave 3[footnote 21]. Announced at Budget 2018, this challenge will build on this to support the transformation of manufacturing, including in life sciences, through digitally-enabled technologies.
Industry are also making new investments in life sciences manufacturing:
- GW Pharmaceuticals, a pioneering UK-based biopharmaceutical company developing and delivering rigorously tested and regulatory-approved cannabis-derived medicines for seriously ill patients, has spent over £20 million expanding its manufacturing facilities in Kent in the past year and more than £100 million in R&D, creating 111 highly-skilled roles, with an additional 98 planned.
- Eli Lilly and Company has committed £5 million to fund research in collaboration with Imperial College London and UCL into the more efficient manufacture of medicines.
Improving the UK environment for businesses to scale up
The first Sector Deal outlined the government’s response to the Financing Growth in Innovative Firms consultation, addressing issues raised in the Life Sciences Industrial Strategy about supporting scale-up with an action plan to drive over £20 billion of patient capital investment over 10 years.
Since the first Sector Deal was published, private investment in UK life sciences has continued to grow: UK biotech companies raised more than £1.5 billion in the first 8 months of 2018, surpassing the 2017’s £1.2 billion total[footnote 22]. Venture capital is particularly strong with £900 million raised.
A full update on the package of measures set out in the first Sector Deal was published alongside Budget 2018[footnote 23]. A particular highlight for life sciences was the launch of the British Business Bank’s new £2.5 billion investment programme, British Patient Capital, and its investment of £9 million in the £250 million Dementia Discovery Fund, a specialist, 15-year venture capital fund that invests in novel science to create new medicines for dementia.
The government also set out a series of new actions that will help address the Life Sciences Industrial Strategy’s recommendation to increase opportunities for pension funds to invest in the sector by addressing regulatory barriers. For example, the government will consult in 2019 on the function of the pension charge cap to ensure that it does not unduly restrict the use of performance fees within default pension schemes. It also announced plans to help pension funds invest in growing UK businesses. Chaired by the British Business Bank, many of the UK’s largest defined contribution pension providers, including Aviva, HSBC, L&G, NEST, The People’s Pension, and Tesco Pension Fund, have committed to undertake a feasibility study to explore options and develop a blueprint for a pooled investment in patient capital.
The sector is also taking action on this issue: to complement the government’s work to give pension funds the confidence to invest in assets supporting innovative firms, the BIA is conducting a detailed analysis of life sciences investment by large institutional investors and private wealth managers to inform a strategic communications campaign to promote the investment and growth opportunities of early and growth-stage companies.
ONI – Next generation super-resolution microscopy
In 2016, ONI was founded as a university spin-out with the aim of making high-end life science research more accessible to every researcher on the planet by miniaturising Nobel prize-winning microscopy technology. ONI has recently completed a significant series A funding round with international investors and leading life sciences patient capital investor, Oxford Sciences Innovation (OSI). This investment has created an additional 100 high value jobs in Oxfordshire and will be used to create further local jobs in the coming years. Such an international investment is a vote of confidence in the UK’s science companies to grow and scale in the UK. In the future, ONI’s technology could have significant diagnostic applications particularly in the cancer space, as it can identify pathology on cells far more accurately than current state-of-the-art techniques used in the clinic.
How do the Life Sciences Sector Deals benefit SMEs?
SMEs comprise 82% of life sciences businesses, over 56,000 jobs (24% of employment) and £7.2 million in turnover[footnote 24]. UK life sciences spin-out companies outnumber the next largest sector (IT & digital) by almost 2 to 1[footnote 25]. This year we highlight:
- HM Treasury’s Patient Capital Review – in response, HM Treasury launched an action plan to drive over £20 million of patient capital investment to finance growth in innovative, high growth potential firms over 10 years.
- As part of this action plan, doubling the annual allowance for investments through the Enterprise Investment and Venture Capital Trust Schemes – after which UK BioIndustry Association’s (BIA) venture capital data for 2018[footnote 26] shows a large increase in fundraising for the sector in the second half of the year.
- Strengthening collaboration between the NHS and innovators through schemes to support innovative SMEs to develop products for the NHS and support to develop real world evidence and access to innovative trial design.
- Supporting the infrastructure needed for growth including: Industrial Strategy Challenge Fund support for Centres of Excellence in digital pathology and radiology; Advanced Therapy Treatment Centres; and, extending the Cell and Gene Therapy Catapult and Manufacturing centre. These programmes will support innovative SMEs to identify, and develop new products, processes and services that can be rolled out across the NHS. SMEs delivering jobs and growth in the UK include Puridify, now part of GE healthcare who opened their new factory in Stevenage in November 2018 and Purolite, with a new factory in South Wales in October 2018 supporting advanced global bioprocessing demand.
NHS innovation and collaboration
The Life Sciences Industrial Strategy set out a vision for a system that has NHS-industry collaboration at its heart, setting a challenge to create an innovation ecosystem that will deliver improved patient outcomes, service efficiency and economic growth, driving the innovation required to address the challenges that the health system faces.
In the first Sector Deal, we set out our initial steps to achieve this by committing to deliver on the recommendations from the Accelerated Access Review and tackle long-standing barriers to innovation in the NHS: a fragmented landscape where innovators find it hard to access support and the NHS has limited visibility of innovations in the pipeline; limited and variable NHS-industry collaboration leading to misalignment of product development and procurement with NHS priorities, or new technologies failing to provide sufficient evidence for uptake; and difficulty getting new technologies into NHS use and spreading what works.
One year on, we have made clear and concrete progress with partners: for example, the NHS is the first health system in Europe to agree access to CAR-T treatments, achieved through the fastest product approvals in the NHS’s 70-year history and supporting our goal to be a world-leading hub for advanced therapies. This success — with a disruptive technology that required a new pathway and route to market — provides a clear signal that the NHS is eager to embrace innovative treatments and can respond with flexibility and agility to adapt its processes, giving patients access to the most effective new treatments faster than ever before.
Across the system we have also seen improvements to the access environment. The average time from marketing authorisation to first NICE output is now 6 months, and to final NICE guidance is now 10.2 months —3.4 months quicker and 5.7 months quicker than 5 years ago[footnote 27]. And, the Innovation Scorecard shows that 77% of the medicines it measures were prescribed more than the previous year[footnote 28].
Industry and government have successfully reached heads of agreement for a new voluntary branded medicines pricing scheme. The new scheme will ensure patients benefit from access to the best available treatment, through faster adoption of clinically and cost-effective medicines, and demonstrates the government’s ongoing commitment to NHS sustainability, health innovation and the UK pharmaceutical sector.
The proposals are expected to include measures to improve uptake of transformative new medicines, as well as commitments from NHS England to provide more proactive uptake support and implementation planning for innovative, cost-effective medicines that provide a significant health gain. This includes committing to achieving uptake levels in the upper quartile of comparator countries for the 5 highest health gain disease classes; more and faster NICE appraisals for new medicines; increased support for small businesses, through exemptions from the cost control mechanism; targeted case management of commercial discussions with NHS England; and greater commercial flexibility for companies that offer the best value new medicines.
In addition to these significant achievements, we have taken the following additional steps to tackle the barriers to innovation:
To simplify and improve the fragmented landscape, we have:
- Established the Accelerated Access Collaborative (AAC) under the leadership of Lord Darzi to improve patient access to innovation. In October, we announced the first 11 products that we will support, potentially improving the lives of 500,000 patients. In early 2019 the AAC will select more products to accelerate through the system.
- Coordinated horizon scanning through ‘HealthTech Connect’, developed by NICE and NHS England to help innovators hold early discussions about market access, and give the NHS a better sense of emerging technologies.
We are strengthening collaboration and co-development between the NHS and innovators:
- £24 million from the Digital Health Technology Catalyst awarded to 26 SMEs is supporting growth in the digital health sector and developing digital solutions the NHS needs.
- £7 million under the NHS Test Bed programme (Wave 2) is helping 7 projects to support real world testing of innovations, and prove their suitability for scaling.
- A new scheme for SMEs has been established, with £1.5 million to design and undertake real-world evidence development and a further £4.5 million available over the next 2 years.
We are improving access to new technologies in the NHS:
- 3 medicines have been appraised and recommended through NICE’s fast-track appraisal process – delivering recommendations on average 4 months faster than standard appraisals.
- The new commercial function at NHS England is increasing the NHS’s capability to engage earlier, and more flexibly, with the life sciences sector.
- We have strengthened Academic Health Science Networks (AHSNs) with a clear mandate to work with industry and local NHS partners to: understand local health and care needs; support system navigation; provide access to testing; and support the adoption and spread of products in the NHS. This has underpinned AHSN engagement with 1,173 companies this financial year leading to 86 long term strategic partnerships for companies with the NHS.
- NHS England’s Innovation Technology Payment is supporting products to overcome procurement and financial challenges, with further products to be agreed in 2018 to 2019.
Progress on industry Sector Deal commitments
One year on from signing, the Orthopaedics Managed Service partnership between Johnson & Johnson Managed Services and Guy’s and St Thomas’ NHS Foundation Trust is delivering results:
- Demand-led supply chain management is improving operating theatre efficiency and freeing up clinicians’ time to be able to focus on patient care rather than operational tasks.
- Improved assets management is reducing procedure cancellations and improving productivity.
- The expansion of current theatre capacity is on track meaning an additional theatre and recovery beds will enable 1,500 additional procedures in 2019.
Johnson and Johnson Medical Devices Companies (JJMDC) has now entered the evaluation phase of its 3-year orthopaedic partnership with Barts Health NHS Trust, and is on track to:
- Realise a target increase in theatre efficiencies of 12%.
- Bring together multidisciplinary teams to agree standardised enhanced recovery pathways for patients.
- Deliver the target of an additional 1,500 bed days to the Trust.
Building a stronger innovation ecosystem
We have made strong progress, but partners across the system agree that there is more to do to meet the ambition of being a world leader in developing and rapidly adopting proven and affordable innovations at scale.
To achieve this, we are embarking on a new programme of work with a much-enhanced and strengthened Accelerated Access Collaborative with a mandate to oversee the innovation landscape for all types of technology:
- Putting the Accelerated Access Collaborative (AAC) at the heart of health innovation — making it the umbrella organisation across the innovation landscape, and the front door to support for innovators and the health system. It will set the strategy and priorities for a more effective innovation ecosystem: overseeing and coordinating health funding and support for innovation, and maximising the opportunity provided by the NHS long-term funding settlement. The AHSNs will be a key element of the AAC and will deliver a consistent, highquality offer to innovators and the health system across the country.
- Improving patient access to more innovations — the AAC will continue to play an important role in identifying transformative innovations, including digital and diagnostic products, for support through the system. It will be given greater flexibility over the number of products it can support, as long as they deliver good value.
- Improving the innovation infrastructure that underpins the AAC’s work through stronger testing infrastructure — we will enable iteration and co-development between the NHS and industry by ensuring that we have sufficient facilities to support product development and pathway testing in real-world settings across multiple locations. This will improve adoption of innovations by giving the NHS greater confidence in the evidence base for new technologies and enabling innovators to be sure their products align with NHS need.
- The government will make funding available to enable NICE to undertake more support for medical devices, diagnostics and digital products. We will tackle the challenge of spreading the best innovations across the system through a new health tech funding requirement for the very best products demonstrating significant value for the health system. This would apply to health tech products, other than pharmaceuticals, which have been assessed as cost saving by NICE. In addition, by 2020, we will significantly increase the number of evaluations of these health tech products conducted by NICE, giving greater scope for considering different types of innovation, including digital products. To support this, NICE will begin presenting guidance and standards as a series of pathways, mirroring the patient experience and the sequence of care clinicians recognise.
Wound care solutions
The wound care industry, working with AHSNs, are pulling together a consortium across the health and care sector to explore how the use of digital clinical decision support tools (like the tool named in the first Life Sciences Sector Deal) can reduce variation and improve patient outcomes and help local areas maximise the impact of their budget and utilise their workforce more effectively. Between them, Smith and Nephew and Convatec are investing over £20 million in UK R&D and academia. Smith and Nephew has already invested some of this money into a pilot with Northumbria Healthcare NHS Foundation Trust to validate this technology and wound care solution in the NHS.
Developing a major new life sciences hub in London
Working with Barts Health NHS Trust, Queen Mary University of London and other partners, the Department of Health and Social Care intends to develop a major new life sciences hub in Whitechapel on land vacated by Barts. We see a real opportunity to support, strengthen and capitalise on the life sciences expertise and potential in the area. Including some space for clinical use by Barts, we plan to develop in the region of 1 million square feet of space, creating exciting opportunities for businesses and property investors. We anticipate total investment in the property to exceed £500 million.
Innovative regulation
An effective regulator, working within well-functioning regulatory frameworks, is an essential part of a strong business environment for the life sciences sector.
The Life Sciences Industrial Strategy highlighted the Medicines and Healthcare Products Regulatory Agency’s (MHRA) global reputation for innovation and leadership in this field. The MHRA’s vision is to be the most forward-thinking regulator for both innovative approaches to regulation and the regulation of innovative new types of products. They have set out how they will support priority areas in the strategy: advanced therapies manufacturing, precision medicine, and furthering our leading position on patient safety, ensuring that the MHRA remains at the global forefront in these areas and meets the needs of industry in the UK.
Supporting advanced therapies manufacturing
As the UK seeks to become a global hub for advanced therapies manufacturing, our regulatory framework must keep pace with developments in the field and provide clarity to industry. Advanced therapies require different methods of manufacturing and delivery to patients. This, coupled with advancements in technologies such as 3D printing, may mean that increasingly medicines and devices will be manufactured in situ close to the patient, and an effective framework to ensure the safety of these products will be essential. In order to provide this clarity, the MHRA will develop a framework for point-of-care manufacture, between April 2019 and March 2021, in collaboration with research councils and the Cell and Gene Therapy Catapult, building on learnings from scientific advice and clinical trial applications.
Leading the way on precision medicine
Advancements in genomic technologies present opportunities for the UK to develop forward-thinking regulation that will operate dynamically for the future. World-leading UK science was pivotal in the development of genomic technologies and precision medicine applications. The UK was the source of the technology on which Illumina, the world leaders in DNA sequencing technology, was founded and now Oxford Nanopore is making a significant impact with its new long-read technology. Maintaining regulatory leadership and expertise in this field is a crucial step in ensuring that the industries developing products based on this science continue to invest in the UK and that UK patients stand to benefit early from new developments.
The MHRA will collaborate with relevant partners to develop a clear UK regulatory pathway for genomic medicines and genomic tests by March 2021, helping to accelerate developments in precision medicine so that treatments can be directly targeted to patients based on their genetic profile. These pathways would apply to, for example, a medicine targeted at a gene-based marker, or aimed at specific gene defects in a disease like cystic fibrosis, with a companion diagnostic. This work aims to produce a roadmap to help industry, academia and healthcare bodies understand which types of clinical trials are most appropriate for their products.
Promoting patient access and patient safety
The MHRA will continue to pioneer fast-tracked and safe patient access to ground-breaking treatments, benefiting both patients through early access to medicines and the sector, as they are able to better gather evidence for the medicines licensing process. It will build on the success of the Early Access to Medicines Scheme, a worldclass scheme helping innovative medicines reach patients quickly and safely, ahead of licensing, with over 1,500 patients benefiting so far.
The MHRA will work with industry and other partners to define a supportive framework for the collection of real-world data during the scheme. This will give industry greater support to gather the evidence they need to underpin licensing applications, in addition to early engagement with MHRA, NICE and the health system. Patients will continue to benefit from earlier access to medicines, often for conditions with unmet clinical need.
Patient safety is paramount to all of MHRA’s work, and data and new technologies present opportunities to further develop world-leading expertise:
- The MHRA will look to assess the feasibility of using artificial intelligence (AI) to identify safety signals in large datasets of health records, enabling real-time identification of issues arising with medicines. In addition to clear patient benefit, industry will better understand how their products work in a real clinical setting.
The MHRA are exploring whether a MHRA Yellow Card Biobank can be developed to capture genetic information from patients experiencing adverse drug reactions (ADRs). This would create a resource to help the MHRA identify where genetics influence who is most at risk from ADRs so that industry and the NHS can reduce these incidents.
Infrastructure
Improved use of high-quality, cost-effective healthcare data presents an opportunity to make the UK the home of data-driven life sciences research, innovation and development – and in so doing, improve outcomes for patients and the NHS.

Digital screen (credit: iStock) [Credit: Getty]
The Life Sciences Industrial Strategy set out how to capitalise on this unique global opportunity.
We are laying down the building blocks to realise the full potential of the scale and diversity of NHS data, while also maintaining public trust in how data is used and maximising the benefits of innovation for NHS patients. We will realise the Health and Social Care Secretary’s tech vision[footnote 29] for a modern technical architecture, underpinned by robust, common standards, that improves services and drives innovation. We want companies of all sizes to set up and grow here on the strength of UK health data, and to make navigating the complex system of access, testing and monitoring of data-driven healthcare as simple as possible.
A lot has happened since the first Life Sciences Sector Deal, with rapid progress on all its commitments. The government is now going further to create the right framework for commercial data agreements, address regulatory challenges and support digitally-enabled pharmaceutical and health tech clinical research.
Ensuring secure and appropriate use of patient data
The first Sector Deal emphasised that improving the UK’s health data infrastructure must be done in a way that keeps data secure; gives patients a choice about how data is used; is transparent about its use and gives confidence that its use was appropriate, secure and legal. The government committed to implement the National Data Guardian’s (NDG) recommendations and has since:
- Led a cross-system programme to embed the NDG’s Data Security Standards through a re-designed Data Security and Protection Toolkit, relaunched in April 2018. This is part of a wider programme, including: £60 million of critical IT infrastructure investment in 2017 to 2018; a new Cyber Security Operations Centre; and an additional £150 million to support cybersecurity in the health and care sector over the next 3 years.
- Launched the national data opt-out service in May 2018, a major milestone in our commitment to build public trust, alongside taking steps to improve accessibility and transparency for patients on data use.
NHS Digital are also delivering on their commitments: a new draft framework[footnote 30], published alongside the Health and Social Care Secretary’s tech vision, sets out core standards on technology and data for all NHS IT systems and digital services to ensure a more joined-up, safer, more efficient system.
The remote data access environment included in the first Sector Deal is live in beta, enabling external customers to remotely and appropriately access data for analysis, with more datasets to be added in 2019. NHS Digital are working with partners to streamline legal and ethical approvals so that they hold more data in trust, meaning easier, secure access for researchers.
Creating the right framework to realise benefits for patients and the NHS where data underpins innovation
Our shared goal, working with partners across the healthcare system and working hand-in-hand with NHS England and NHS Improvement, is to improve outcomes for patients, make the NHS more efficient and cost effective, and make the UK home to the latest data-driven scientific advances in healthcare. Our starting point is that the government wants NHS partnerships to flourish within the strictest parameters of transparency and accountability. We recognise that each partnership will be different, and that there is no ‘one-size-fits-all’ solution. Some datasets may increasingly be made openly available to enable innovators to develop solutions to some of our greatest healthcare and technology challenges, but we also anticipate that NHS organisations will enter increasing numbers of commercial arrangements, where it is right that NHS patients benefit directly from the innovations enabled using NHS data.
We recognise that NHS organisations need expert guidance in reaching these types of commercial agreements. The Office for Life Sciences has already begun detailed policy work to consider the different commercial models that could be appropriate and what kind of national guidance and hands-on commercial support NHS organisations will need. We know that these are complex issues which generate different views and we want to hear from patients, the public and partners on whether these 5 guiding principles are the right basis for the more detailed national policy framework:
- Principle 1: Any commercial use of NHS data must have an explicit aim to improve the health and care of patients in the UK, including through the discovery of new treatments, diagnostics, and other scientific breakthroughs. Where possible, the terms of any arrangements should include quantifiable and explicit benefits for UK patients which will be realised as part of the arrangement.
- Principle 2: NHS data is an important asset and, in entering into commercial arrangements, NHS organisations should ensure they agree mutually-beneficial and fair terms. In particular, the boards of NHS organisations should consider themselves ultimately responsible for ensuring that any arrangements entered into by their organisation are mutually beneficial and fair.
- Principle 3: Any commercial arrangements agreed by NHS organisations should not undermine, inhibit or impact the ability of the NHS, at national level, to maximise the value or use of NHS data. NHS organisations should not enter into exclusive arrangements, nor include conditions limiting any benefits from being applied at a national level, nor undermine the wider NHS digital architecture, including open standards and interoperability.
- Principle 4: Any commercial arrangements agreed by NHS organisations should be transparent, clearly communicated, and not undermine public trust and confidence either in the NHS or wider government data policies.
- Principle 5: Any commercial arrangements agreed by NHS organisations should fully adhere to all national level legal, privacy and security obligations, including in respect of the National Data Guardian’s Data Security Standards.
In parallel, we are developing the concept of a national centre of expertise which would oversee the policy framework, provide specialist commercial and legal advice to NHS organisations entering commercial agreements, develop standard contracts and ensure that the advantages of scale in a single-payer health system deliver benefits for UK patients. The centre would be expected to work closely with the Centre for Data Ethics and align with the National Data Strategy.
The Department of Health and Social Care intends to publish more detail on the emerging policy framework, including any required alignment with the Initial code of conduct for data-driven health and care technology[footnote 31], and the structure and responsibilities of the national centre in the first quarter of 2019 following detailed discussions with patients, the public and partners.
Supporting the development of measures to improve the UK’s health data infrastructure
As previously highlighted, the UK’s health data assets provide an exceptional opportunity to lead in both pharmaceutical and health tech discovery and clinical development, and also to greatly enhance the functionality of the NHS.
These assets have been significantly expanded and enhanced thanks to increasing digitisation of the system and increased interoperability, based on technology improvements.
The government is continuing to drive this, with a £475 million digitisation package, announced by the Health and Social Care Secretary, to accelerate the roll-out of innovative technology aimed at improving care for patients and to support staff to embrace technology-driven health and care. This builds on the Global Digital Exemplar programme – backed by a further £200 million of government funding in September 2018 – where NHS providers who have reached globally-recognised levels of digital maturity share their learning on delivering exceptional care, efficiently, through the use of world-class digital technology and information with other trusts.
The unique feature of UK health data resource is its longitudinal nature, covering individuals across the system from birth to death in both primary and secondary care. Along with its sheer scale, this provides the UK with one of the most powerful health datasets in the world.
A large number of national data assets are hosted through NHS Digital, for example the Health Episodes Statistics (HES), a comprehensive, coded dataset on all hospital admissions. Other major data assets include: primary care records; registries such as the Cancer Registry managed by Public Health England; and unique, specialised datasets such as Genomics England’s. In addition, the UK has significant assets in consented populations from its large cohorts, particularly UK Biobank, and disease-specific charities, which often manage significant registries of patients.
The government is investing to further enhance the UK’s health data capabilities through complementary, well-aligned programmes that will improve connectivity and interoperability across datasets, in line with the Life Sciences Industrial Strategy. This includes:
- Health Data Research UK (HDR UK) has announced a Strategic Partnership Agreement with NHS Digital to ensure joined-up alignment of data ecosystem initiatives, such as NHS Digital’s Data Processing Services, Digital Innovation Hubs and the National Clinical Trials Platform.
- HDR UK is also working with NHS Digital to establish a UK Health Data Research Alliance to encourage widespread access to health data in a trustworthy, ethical and secure way. As the Alliance forms, members will work together to support and promote a common set of behaviours around responsible data sharing for research and innovation.
- A £43 million investment to enhance NHS Digital’s core data services, including more automated processing, less movement and replication of data, and the utilisation of modern solutions on the cloud. This will increase security, reduce the cost and increase the efficiency of NHS Digital services, while continuing to fully respect citizens’ data rights.
- Funding to further develop Clinical Practice Data Link (CPRD) to enable secondary care data flows from hospitals to support specific real-world clinical and patient-consented studies in combination with CPRD primary care data, which will achieve even greater efficiencies in delivering real-world clinical trials across the health care spectrum.
- 5 Local Health and Care Record Exemplars, selected by NHS England and supported by £37.5 million national funding alongside local investment, to further integrate patient records between primary, secondary and social care. The addition of a second cohort of health and care systems will provide 63% coverage of the English population.
- The creation of Digital Innovation Hubs announced in the first Life Sciences Sector Deal. Backed by £37.5 million of UKRI Industrial Strategy Challenge Funding, Health Data Research UK has now been appointed to deliver the programme, aligned with its wider activity to create a UK-wide infrastructure and common framework for the use of data for research and innovation. HDR UK is running a sprint innovation competition to identify exemplars which could test the technical solutions required for Hubs. A period of detailed design and dialogue with stakeholders on the Hubs will then be followed by a full specification and UK-wide call for applicants in May 2019. 3 to 5 regional and interoperable Digital Innovation Hubs will be selected to further integrate health data assets both regionally but also in collaboration with NHS Digital across the country.
Alongside improving patient care, a major focus of this work is on supporting and expanding digitally enabled clinical research so that both clinical and real-world data studies can be greatly facilitated, uniquely positioning the UK to take advantage of this digital revolution.
Under the Strategic Partnership Agreement announced between NHS Digital and HDR UK, Professor Martin Landray will lead work on the creation of data services to support a 21st century clinical trials platform. It will focus on the creation and linkage of national data systems to support rapid, efficient trials that meet the needs of regulators and NHS patients, while protecting individuals’ data rights. This includes a system for assessing the feasibility of recruiting well-defined patient populations into trials, with a vision to demonstrate an efficient and industry-friendly, England-wide clinical trials service for feasibility and eligibility by autumn 2019 to complement the NIHR Clinical Research Network Study Support Service.
In addition, over the next year, the government will explore the potential of patient registries with stakeholders like the NHS, PHE and the Association of Medical Research Charities (AMRC), who have already published a report on this subject[footnote 32], to understand whether there is untapped potential which could be better utilised by the sector.
NHS Digital and NHS England, working with research partners including the NIHR, will deliver new services via the NHS App from autumn 2019 to enable patients to become more directly engaged in clinical research relevant to them, by registering their willingness to participate and indicating their preferences for types of research. Crucially, subject to consent, it should be possible to obtain relevant data from the digital records of patients enrolled in research, reducing costs and improving efficiency, compared to conventional, labour-intensive methods. Along with digitally-enabled recruitment, this would dramatically reduce the cost and speed of recruitment to and running of large-scale clinical studies, particularly for late-stage clinical trials, the most costly component of the drug discovery process. By 2020, the NHS app will also be a channel to provide participants with information about the outcome of the research they were part of. By 2021, it will allow people to upload data from their wearables and lifestyle apps, swiftly, safely and securely, and consent for it to be linked with their health records, in order to help improve NHS patient care through research.
The ORION-4 Trial on a new cholesterol-lowering drug
The ORION-4 trial, a partnership between the University of Oxford’s Clinical Trial Service Unit, its Epidemiological Studies Unit and The Medicines Company, aims to recruit 15,000 participants, including 12,000 in the UK, for a trial of a new cholesterol-lowering investigational drug given just once or twice a year. In an NHS Digital pilot, following advice from data governance experts, the team accessed national health data for England to identify 1 million eligible participants across 50 hospitals. This provided them with faster, earlier assurance on the feasibility of the trial, identified a more diverse range of sites than usually possible for large-scale trials, and enabled a wider, representative cross-section of the UK population at risk of cardiovascular disease to participate. Thoughtful design and effective use of digital tools mean the trial is due to be completed on time, at high quality, for a fraction of the cost of similar recent trials.
Creating a regulatory framework fit for the future
The UK regulatory regime needs to be fit-for-purpose and keep pace with rapid technological developments so that patients, clinicians and care professionals can access the best possible treatments quickly and safely, and innovators are supported to develop health and care solutions in safe spaces.
Clarifying existing frameworks and how they work for digital technology will be essential if we are to create the right conditions for innovation.
The first iteration of the code of conduct for data-driven health and care technology provides clarification on what is expected from our technology partners, in terms of commercial arrangements and standards, to ensure that technology firms are able to quickly and efficiently deliver the best possible outcomes for patients and the system. We are working with a number of technology firms, pharmaceutical companies, national funders and medical charities who have agreed to incorporate the principles from the code of conduct in their work[footnote 33]. We will continue to engage with stakeholders to ensure that the code continues to be fit-for-purpose and supports innovation. We expect to publish an updated version of the code in due course.
The MHRA recognises the need to keep pace with the opportunities offered by data-driven technologies, specifically algorithms and AI. For example, developing the necessary structures to deal with learning algorithms that continuously change based on a continual flow of data.
To harness these opportunities, the MHRA has successfully bid into the Industrial Strategy’s ‘Regulator’s Pioneer Fund’ for £740,000 to work with NHS Digital on a proof-of-concept pilot to develop synthetic datasets that mimic real-world data to validate algorithms, including AI algorithms used in medical devices. The pilot will invite a small group of innovators to validate their algorithms against the synthetic datasets. If successful, the synthetic datasets will be made available to innovators and act as a regulatory sandbox to help with product validation. This will help make the UK an excellent place for AI and diagnostic companies to develop and test their products, and help develop an effective route to market for innovations.
Places
Over half the UK life sciences workforce is outside the south east cluster[footnote 34]. Last year the first Life Sciences Sector Deal committed to develop more regional approaches in future phases of work.
Illustration of the world (credit: Adobe Stock ID:19721654) [Credit: Adobe Stock]
We are now setting out new commitments in this regional component of the deal, as well as casting a spotlight on the full breadth of UK activity, demonstrating the sheer scale of opportunity.
The government is investing in place-based approaches and has launched the Strength in Places fund, a new approach to research and innovation funding. The Local Industrial Strategy programme is well underway with the West Midlands, Greater Manchester and Thames Valley publishing progress reports highlighting their life sciences strengths.
With so much economic potential, we are taking action to help market regional strengths. In response to industry feedback, we are renewing the government’s offer of Life Sciences Opportunity Zone status, so that areas of existing or emerging strength can apply for the designation to help raise their profile at international level. The existing Opportunity Zone is the Charnwood Campus which, since its designation in 2016, has become a focal point for life sciences in the Midlands. Applications will be considered from existing or new UK life science locations, such as science or manufacturing parks, incubators and accelerators that demonstrate a high level of commitment to attract companies and support growth.
The sector is already spotting potential opportunity zones and Bruntwood, the owner of Alderley Park, in partnership with Legal & General Capital, have announced a deal that sees the 2 partners invest £360 million of capital, property and intellectual assets to create the UK’s largest portfolio of science and technology assets. Initially expanding within the Northern Powerhouse and Midlands Engine regions, Bruntwood SciTech partnership seeks to make the most of untapped potential in the UK’s science and technology sector. With a plan to create over 20,000 high-value jobs over the next 10 years, the deal represents the largest investment made in science and technology property assets in Europe this year.
Within each region, self-assembled, place-based organisations act as multi-faceted convening bodies to bring together the breadth, value, and expertise across the health and life sciences supply chain to drive economic growth. Over the next year the government, led by the Department of International Trade and the Office for Life Sciences, will continue to work with our Life Sciences Champion, Professor Sir John Bell, in collaboration with the leading UK cluster organisations (including Health Science Scotland, Northern Health Science Alliance (NHSA), MedCity, Midlands Innovation, the GW4 Alliance, Life Science Hub Wales and Life Sciences Northern Ireland) and national centres of excellence, to make the landscape easier for investors to navigate and support growth.
The most well-known cluster in the UK, referred to as the golden triangle of Oxford, Cambridge and London, is extremely strong and rightly has a reputation for being the best place in the world to do discovery science. In the first Life Sciences Sector Deal we highlighted government investments in local infrastructure in the Cambridge-Milton Keynes-Oxford corridor to ensure the area’s continued growth. In the 2018 Budget, the government has gone further, announcing £20 million to further develop the plan for the critical central section of East-West rail between Oxford and Cambridge. Other thriving UK clusters are growing, based on strong government and sector partnerships, and are showcased here.
The distribution of the UK’s vibrant life sciences industry[footnote 35]
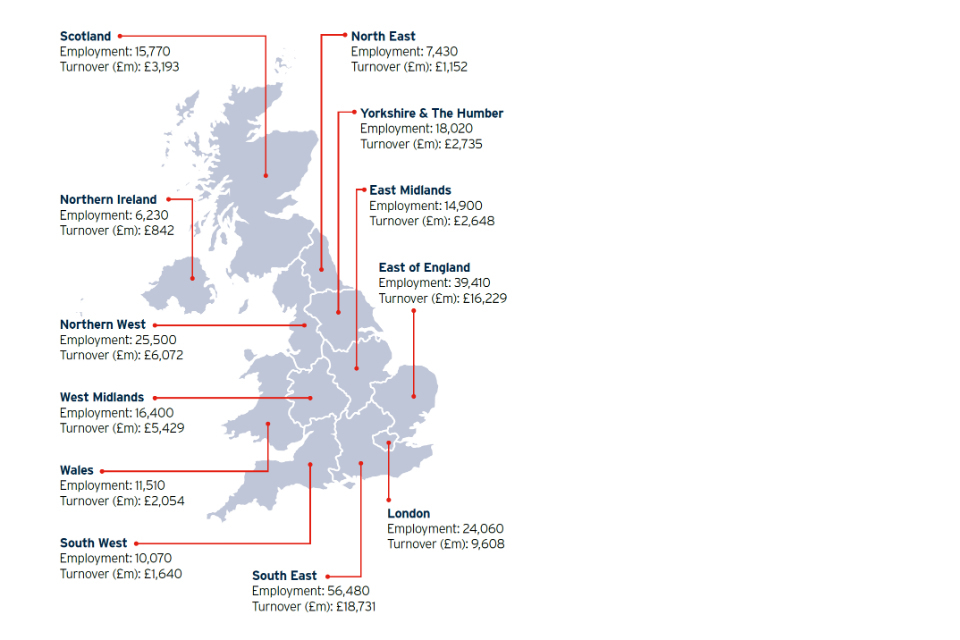
Map showing the distribution of the UK’s vibrant life sciences industry
Thames Valley
The Thames Valley is at the heart of the golden triangle and is home to the highest concentration of disruptive technology and AI companies in the UK[footnote 36]:
- In the Thames Valley, industry, academia and medical research institutions, under the umbrella of the Thames Valley Life Sciences Partnership, chaired by Bayer, are collaborating on data analytics and artificial intelligence to develop a ‘global artificial intelligence and imaging hub’ to develop next generation targeting, going across a range of therapy areas, to identify undiagnosed and suboptimally treated patients.
- At Thames Valley Science Park in Reading, the University of Reading is developing a strong cluster of R&D focused health life sciences companies. Opened in March 2018, the park will be amongst the biggest dedicated science parks in the region and one of the largest in the South East. Funded by a £35 million investment from the University of Reading and the European Regional Development Fund, the investment has enabled the provision of office and laboratory space for around 20 companies, from start-ups to global research and development centres. This is the first step in the establishment of a significant new Science Park which, once complete, will have the potential to provide up to 5,000 new jobs.
- Proton Partners International has recently completed a £30 million cancer treatment centre – ‘The Rutherford Cancer Centre, Thames Valley’ on the park which includes high-energy proton beam therapy.
The Northern Powerhouse
The Northern Powerhouse is home to 21% of the total UK life science sector workforce, and has grown by more than 9% to over 50,000 employees since 2012. Latest figures for 2017 show the value of this sector in the North to be over £13.6 billion[footnote 37]. With £1.6 billion of planned investment in the pipeline over the next 5 years put forward by the Northern Health Science Alliance in response to the first Life Sciences Sector Deal, it is clear that this is a significant, growing cluster.
Investments include:
- Fujifilm Diosynth in Tees Valley has invested £5.7 million this year, building on a £6.7 million investment made in 2017 in high throughput process development of monoclonal antibodies, which will support its growing portfolio of projects to develop and manufacture life-changing medicines for its clients.
- Liverpool and Northumbria are now home to 2 of Proton Partners International Rutherford Cancer Centres with an initial investment of £70 million, with Rutherford Diagnostics recently announcing a £15 million diagnostic centre in the Liverpool Knowledge Quarter.
- QIAGEN are working with Health Innovation Manchester to develop a world-leading genomics campus in the heart of Manchester’s health innovation district. The new Genomic Health Innovation Campus aims to attract a cluster of companies to Manchester to drive pioneering research and development. The investment will create 250 new jobs while supporting more than 1,000 jobs indirectly in the Manchester corridor.
- The Leeds Medtech Hub has secured £70 million towards its £250 million investment target and its Nexus Centre is nearing completion. Alongside this, £15 million has been secured to deliver connected electronic patient records and a further £9.5 million to back Grow Medtech and its 20 new partners who will support work to deliver early stage medical technology innovation.
- At Sheffield Olympic Legacy Park, good progress has been made on plans for an Orthopaedic and Rehabilitation Research and Innovation Centre and a Centre of Child Health and Technology.
The Midlands Engine
The Midlands is the manufacturing heart of the UK, one of the largest clinical trials clusters in Europe and host to impressive health data platforms. The region integrates industry (particularly SMEs) with local government, universities and the NHS to deliver new technologies, tests and treatments in a stable but ethnically diverse population of 11 million.
- Birmingham Health Partners raised more than £100 million during 2017 to 2018 to expand initiatives in genomic medicine, diagnostics, clinical trials, healthcare informatics and medical technologies underpinning the strategy for development of the new £300 million Birmingham Life Sciences Park.
- During 2018, Boehringer Ingelheim has partnered with the Leicester Diabetes Centre and the Leicester Real World Evidence unit to design and deliver high-quality, real-world studies. This is a prime example of the Midlands’ cutting-edge, data science-enabled ecosystem that is delivering large, collaborative, NHS partnering innovation platforms.
- Supported by strong Midlands partnerships, the new £300 million Defence and National Rehabilitation Centre, also awarded £70 million from government, the NIHR Trauma Management MedTech Cooperative and Medical Device Testing & Evaluation Centre are driving development of technologies such as robotic exoskeletons and scar-reducing wound care products.
- In 2017 to 2018 the Institute of Translational Medicine leveraged over £44 million in new research and clinical innovation funding across industry, philanthropy and public bodies to accelerate precision medicine studies and clinical trials.
The Great West
The Great West region has a fast-growing life science industry presence, realised by the sector’s synergy with more mature regional technology and digital businesses - through convergence with AI, high-performance computing and quantum technologies, a flourishing SME ecosystem and world-leading academic expertise in biodesign, genomics and epidemiology, and quantum science. This is exemplified by:
- NovoNordisk acquired University of Bristol spin-out Ziylo Ltd in August 2018 in a staged acquisition with a potential deal value that could exceed £640 million ($800 million) and has entered into a collaboration with Carbometrics, based at fastgrowing incubator facilities, UnitDX.
- Charles River acquired University of Bristol spin-out KWSBiotest, a leading Contract Research Organisation specialising in immunology oncology discovery testing services, in January 2018. This milestone-based £18 million ($24 million) investment cements a strong service provider presence in the region.
- External investment of around £90 million has been made into world-leading quantum technologies research at the University of Bristol. The University will establish a new £43 million Quantum Technology Innovation Centre to accelerate life sciences industrial impact in 3 areas: (i) improving the speed of genomic data processing and drug discovery; (ii) quantum enhanced sensing and imaging for diagnostic devices and cell biology; and (iii) quantum communication for data security, for example medical records.
Northern Ireland
Northern Ireland is enjoying a growing international reputation in key areas such as diagnostics, precision medicine and advanced manufacturing:
- Randox Laboratories is leading a £50 million investment in 3 new R&D centres of excellence in clinical diagnostics in Northern Ireland. Randox is partnering with Queen’s University Belfast and Ulster University, and committing £27 million to the project, supported by a further £23 million in government support from Invest Northern Ireland. Randox, who have invested over £300 million to date in their proprietary Biochip Array Technology, aim to drive significant improvements in healthcare through earlier, more accurate diagnosis.
- Almac Discovery, a research-driven drug discovery company, is making a £15 million investment in a Centre for Precision Therapeutics to develop new therapeutic approaches for diseases of unmet medical need, in collaboration with Queen’s University Belfast and supported by £5.4 million of government funding.
- Northern Ireland is a centre of expertise in digital pathology: Philips computational digital pathology capability is in Belfast and will be heavily involved in delivering 2 of the new Industrial Strategy Challenge Fund centres of excellence for digital pathology with AI. Invest NI has recently awarded Philips a grant of £1.3 million towards a £3.6 million investment to develop the next generation of its AI-enabled digital pathology platform. Queen’s University Belfast’s new £10 million Precision Medicine Centre of Excellence, supported by £5.8 million from government, will aim to improve treatment for cancer patients and develop an internationally-accredited clinical laboratory focusing on diagnostics, bringing together genomics, digital pathology and big data analytics.
Wales
Wales has a whole-system approach to health and social care, underpinned by a national digital infrastructure. It is a global leader in value-based healthcare and tracking outcomes. This is linked to economic development through projects like the £200 million Wellbeing Village, which makes Wales an ideal partner system for rapid adoption and evaluation of new medicines, technologies and services. Key projects include:
- Through a partnership that brings together EU, Welsh government and academic investment of £24 million, the ACCELERATE Programme, led by the Life Sciences Hub Wales will focus on accelerating the exploitation of innovation and technology opportunities that meet needs across the health and social care system.
- The UK Dementia Research Institute at Cardiff is a £20 million research centre aimed at finding effective treatments for dementia, with more than 70 global scientists and plans to grow to 100. The centre is 1 of 6 that make up the UK Dementia Research Institute (UK DRI) with a total £290 million investment. It will build upon groundbreaking scientific research strengths in dementia genetics; immunology; computational analytics; and neuroimaging to identify disease mechanisms and therapies. This will identify new targets for drug development, develop new approaches for delivering effective clinical trials and attract industry partnerships to develop new therapeutics and diagnostics.
Scotland
Scotland’s life sciences community is working closely with NHS Scotland to develop an ecosystem which supports growth and development within the sector. The unique Scottish Health Research and Innovation Ecosystem is a portal which will facilitate collaborations between Scottish businesses and the NHS. Major collaborations include:
- The new Industrial Centre for AI Research in Digital Diagnostics (iCAIRD) was announced in November 2018. With its hub in the Glasgow Clinical Innovation Zone, this collaboration from NHS Scotland, academia and industry is expected to incubate more than 40 companies, generating over 500 highly-skilled, sustainable jobs over the next 10 years of the project.
- Industry partners, Canon Medical and Philips, and innovative SMEs, such as Bering, Cytosystems Ltd, Deep Cognito Ltd, Glencoe Software, Intersystems, Kheiron Medical Technologies and Nvidia, have together invested over £5 million. Collaborating with research-active clinicians, they will combine to solve healthcare challenges and create an effective route to market.
- The University of Strathclyde worked with Biogelx Limited and supported by the Innovation Centre – Scottish Centre for Sensors and Imaging Systems (CENSIS), developed a bio-imaging platform which can perform accurate, live, intracellular analysis in 3D. This project addressed a need within various cell markets but is particularly key for stem cell research, impacting on areas such as tissue engineering and cell therapy.
People
Highly-skilled workers are at the heart of the Life Sciences Industrial Strategy and the first Sector Deal set out initial measures to further develop the home-grown skills the sector needs, whilst also ensuring it can access talent from around the world. The government and the sector are now going further in their commitments.

Doctors looking at a screen (credit: Philips Healthcare) [Credit: Philips Healthcare]
Increasing uptake of life sciences apprenticeships
The Life Sciences Industrial Strategy noted that apprenticeships will play an increasing role in addressing industry’s need for highly-skilled employees. A survey by the ABPI[footnote 38] found a 31% increase in apprenticeships since 2015, up by 169% since 2013 — this increase being primarily higher-level apprenticeships — showing how they are increasingly seen as a viable option to more traditional academic routes. In the first Sector Deal, government committed to work with the Institute for Apprenticeships (IfA) to prioritise the development of standards for life sciences.
Since then, through the IfA’s ‘Faster and Better’ programme, industry trailblazer groups developing standards have received specialist support from a dedicated relationship manager to guide employers through each step of the process. The IfA’s Health and Science route panel (formed of industry leaders) has worked with 20 life sciences trailblazer groups to deliver standards in priority areas for the Life Sciences Industrial Strategy, for example clinical trials, bioinformatics, and regulatory affairs.
The government is now taking further action on the levy in response to ongoing discussions with industry, helping to address an issue raised in the Life Sciences Industrial Strategy and the Science Industry Partnership’s (SIP) Apprenticeships survey[footnote 39]. Action includes measures announced at Budget 2018:
- Larger employers highlighted they would value the ability to transfer more levy to SMEs in their supply chain to support the development of a broader life sciences ecosystem. Budget 2018 announced that this would increase from 10% to 25% from April 2019. Up to £90 million will be made available in 2019 to 2020 to fund the costs of apprenticeships supported by transfers.
- SMEs will be further supported by a £240 million commitment in Budget 2018 to help reduce the amount that smaller employers have to contribute to training new apprentices from 10% to 5% in 2019.
However, the government recognises that there are specific issues affecting the life sciences sector, particularly SMEs, that could mean barriers remain to their uptake of apprenticeships. We are therefore announcing our intention to work with UKRI, DfE and the Education and Skills Funding Agency to explore the potential for a pilot programme to better enable SMEs in the life sciences sector to take on apprentices.
SIP is delivering on its commitment in the first Sector Deal to roll out regional approaches to meeting industry skills needs, particularly on apprenticeships uptake: following the successful set-up of SIP Cambridge, it is looking to extend this approach to SIP Liverpool.
Increasing uptake of science, technology, engineering and maths (STEM)
The Life Sciences Industrial Strategy recommended taking steps to further strengthen UK STEM education.
The government has established a new, Department for Education-led STEM External Working Group to feed views and expertise from industry into cross-government work, operating as a working group to test current and future thinking on STEM. The group comprises major stakeholders across STEM industry representation bodies, academic institutions and employers, and includes membership from the life sciences sector, ensuring their views are well represented.
Industry is also developing innovative new solutions to encourage young people to take up and pursue STEM subjects and careers:
- The Association of the British Pharmaceutical Industry (ABPI) will support the British Science Association’s work to deliver a new government-funded competition for young people, inspiring them about STEM through learning how society, technologies and jobs will change as a result of addressing the Industrial Strategy Grand Challenges.
- The ABPI will convene key partners in healthcare and industry to identify opportunities to improve support focused on research and innovation for medics throughout their training and career, for example developing the ABPI’s careers resource into a new joint portal that will support medical students, amongst others, with their career.
- The ABPI will also work with Health Education England to align industry’s support for work on medical careers with the Topol Review, which will make recommendations next year on how to prepare the healthcare workforce to deliver the digital future.
Accelerating convergence across life sciences and other disciplines
The Life Sciences Industrial Strategy highlighted enormous opportunities for innovation at the interface between life sciences and other disciplines, recommending funding to facilitate cross-disciplinary and cross-sectoral working and the exchange of ideas.
New UKRI programmes are helping to deliver this:
- Clinical academic research partnerships: A new pilot scheme will be launched to enable research-qualified, but not research-active, NHS consultants to participate in collaborative high-quality research partnerships, facilitating engagement and better connecting basic biomedical science to clinical research. The scheme will be launched in late 2018, supported by £10 million from the Medical Research Council.
- Centres for Doctoral Training in Artificial Intelligence: A UKRI-wide call was opened earlier this year for universities to apply and will support up to 950 new students over 5 years, some of which will be aligned with life sciences.
Building a 2030 Skills Strategy
The Life Sciences Industrial Strategy recommended the development of a skills action plan based on a gap analysis of key skills for sciences.
The Science Industry Partnership (SIP), with key partners including the Association of the British Pharmaceutical Industry and the UK BioIndustry Association, will lead and deliver a Life Sciences 2030 Skills Strategy, funded by £100,000 from SIP, with further funding from trade association partners and the government. It will build a clear evidence base of the status of life science skills and future scenarios to 2030, focusing on medicines manufacturing for established medicines and advanced therapies (supported by the Medicines Manufacturing Industry Partnership), as well as other emerging technologies, such as AI, and to identify what is needed in addition to current provision.
Implementation
Governance
The Life Sciences Council has been established as the most senior strategic partnership forum between the government and the sector to provide oversight of the future of UK life sciences, including delivery of the vision of the Life Sciences Industrial Strategy and the Life Sciences Sector Deals. Chaired by the Secretaries of State for Business, Energy and Industrial Strategy and for Health and Social Care, along with the Chair of the British Pharmaceutical Group for industry, the Council consists of global industry and charity CEOs representing the full breadth of the sector; the Chief Executive of NHS England; the government’s Life Sciences Champion; and senior representatives of relevant government funding organisations and policy leads.
The Council is supported in its strategy and oversight role by the Life Sciences Industrial Strategy Implementation Board, chaired jointly by the government and industry. It includes senior representatives from the government, the NHS, industry and charities.
The Board meets quarterly to review progress on sector deal commitments and to consider wider implementation of the Life Sciences Industrial Strategy’s vision, for example, advising on the proposals for new commitments made in this second Sector Deal.
Metrics
To monitor the overall impact of the first Life Sciences Sector Deal, we have worked with our delivery partners through the Life Sciences Industrial Strategy Implementation Board to develop metrics for each of the projects included in the first deal, in addition to a set of overall success metrics based on the expected long-term impacts of each individual project:
- Increased investment in the UK life science industry and an increase in UK GDP
- Growth of UK Life Science companies
- Improved patient access and uptake of cost-effective innovations[footnote 40]
- Increased investment in clinical research and increased share of clinical research participation
To be able to monitor and evaluate long-term impact we have used a combination of existing annual targets, forecasting and current trends in the data to create the baselines which will help us evaluate our performance.
Since many of the projects are currently in early stages of implementation, we expect to monitor this data over a period of years to be able to see a long-term impact. As well as the overall success metrics, delivery partners will also evaluate individual projects and their impacts on the industry, using the specific metrics developed on a project-by-project basis. New metrics to measure the impact of new commitments in this second Life Sciences Sector Deal will also be developed with the Life Sciences Implementation Board. Some of the high-level life sciences metrics collected will be reported to evaluate the Industrial Strategy Sector Deal programme as a whole.
References
-
Office for Life Sciences (OLS), Strength and Opportunity 2017: the landscape of the medical technology and biopharmaceutical sectors in the UK (2018) ↩
-
Professor Sir John Bell, Life Sciences Industrial Strategy: a report to the government from the life sciences sector (2017) ↩
-
Office for Life Sciences (OLS), Industrial Strategy: Life Sciences Sector Deal (2017) ↩
-
Department of Health and Social Care (DHSC) & Department for Business, Energy and Industrial Strategy (BEIS), Making a reality of the Accelerated Access Review: improving patient access to breakthrough treatments (2017) ↩
-
HM Treasury, Financing growth in innovative firms: consultation response (2017) ↩ ↩2
-
Subject to business case approval ↩
-
Department of Health and Social Care (DHSC), Prevention is better than cure: our vision to help you live well for longer (2018) ↩
-
Calculated by the Cancer Intelligence Team, Cancer Research UK. Based on current distribution of stage at diagnosis for cancers with a recorded stage of disease in England (obtained from PHE), cancer incidence projections for 2033 and estimates for 5 year cancer survival by stage. Contact cancerintelligence@cancer.org.uk for more information. ↩
-
Subject to business case approval ↩
-
Association of the British Pharmaceutical Industry (ABPI), Number of Phase 1 trials - global (2016) ↩
-
National Institute for Health Research (NIHR), Key statistics 2017-18 (2018) ↩
-
NHS England, 12 actions to support and apply research in the NHS (2017) ↩
-
National Institute for Health Research (NIHR), Performance in initiating and delivering clinical research (2018) ↩
-
HRA and MHRA, The combined ways of working pilot (2018) ↩
-
Department for Business, Energy and Industrial Strategy, Industrial Strategy: building a Britain fit for the future (2017) ↩
-
Office for National Statistics (ONS), Business enterprise research and development UK (2017) ↩
-
This is subject to finalising a suitable site and contractual negotiations ↩
-
Medicine Manufacturing Industry partnership, Advanced Therapies Manufacturing Action Plan: Retaining and attracting advanced therapies manufacture in the UK (2016) ↩
-
Cell and Gene Therapy Catapult, Annual Review (2018) ↩
-
Sources: The number of companies drawn from the SBC and catapult websites. The financial figures drawn from publicly available investment information collated by SBC. Job numbers from: Building the world’s most complete advanced therapies ecosystem: Annual Review 2018 ↩
-
Subject to business case approval ↩
-
UK BioIndustry Association, Biotech Financing Update (2018) ↩
-
HM Treasury, Financing growth in innovative firms: one year on (2018) ↩
-
Office for Life Sciences (OLS), Strength and Opportunity 2017: the landscape of the medical technology and biopharmaceutical sectors in the UK (2018) ↩
-
Spinouts UK, Spinouts UK annual report 2017 (2017) ↩
-
UK BioIndustry Association, Biotech Financing Update (2018) ↩
-
Office for Life Sciences (OLS), Life Science Competitive Indicators, 2018 (2018) ↩
-
NHS Digital, NICE Technology Appraisals in the NHS in England (Innovation Scorecard) (2018) ↩
-
Department of Health and Social Care (DHSC), The future of healthcare: our vision for digital, data and technology in health and care (2018) ↩
-
NHS Digital, BETA: NHS digital, data and technology standards framework (2018) ↩
-
Department of Health and Social Care (DHSC), Initial code of conduct for data driven health and care technology (2018) ↩
-
Association of Medical Research Charities, Saving lives with patient data registries (2018) ↩
-
This includes: IQVIA, Kheiron Medical, Great Ormond Street Drive Unit, Babylon Health and Genomics England ↩
-
Office for Life Sciences (OLS), Strength and Opportunity 2017: the landscape of the medical technology and biopharmaceutical sectors in the UK (2018) ↩
-
Office for Life Sciences (OLS), Strength and Opportunity 2017: the landscape of the medical technology and biopharmaceutical sectors in the UK (2018) ↩
-
TechNation and TechCity, Growth of the UK’s digital tech sector (2015) ↩
-
Office for Life Sciences (OLS), Strength and Opportunity 2017: the landscape of the medical technology and biopharmaceutical sectors in the UK (2018) ↩
-
Association of the British Pharmaceutical Industry (ABPI), Apprenticeships hit 4-year high in pharmaceutical industry (2018) ↩
-
Science Industry Partnership (SIP), SIP Apprenticeship Survey 2018 (2018) ↩
-
NHS data refers to England only ↩
