UK COVID-19 vaccines delivery plan
Updated 13 January 2021
Ministerial foreword
We have always known that vaccines would be our best way out of this pandemic and towards a more normal way of life. It is why we moved fast and early: supporting ground-breaking research from January last year, and pre-ordering millions of vaccines on behalf of the whole of the UK. As a result of this work, we are the first country in the world to authorise a vaccine against COVID-19.
Today we have 3 authorised vaccines for COVID-19; more than any other country in the world. Our priority is to save as many lives as possible, as quickly as possible, while also reducing the hospitalisations that are creating such pressure on the NHS.
Across the UK, we have already vaccinated over 2 million people, and are on track to deliver on our commitment to offer a first vaccine to everyone in the most vulnerable groups by the middle of February. These groups have so far accounted for 88 per cent of COVID-19 fatalities. Then, we will expand the programme so all adults can be vaccinated by the autumn.
This vaccine delivery plan sets out how we can do that.
It is the culmination of months of preparation and hard work by the Vaccine Taskforce, the NHS, Public Health England (PHE), our research institutions and Armed Forces, and local and regional government at every level. International collaboration has been critical. Here at home, we have been putting the architecture in place to deliver hundreds of thousands of vaccines per day – from small GP-led sites to large vaccination centres in football stadiums.
We’re under no illusion as to the scale of the challenge ahead. In more normal times, the largest vaccination programme in British history would be an epic feat. But against the backdrop of a global pandemic – and a new more transmissible variant – it is a huge challenge. Yet, we have never felt more confident we can achieve our ambitious goals.
As our NHS heroes put this vaccine delivery plan into action, and until we can see vaccines are saving lives, we must all play our part. We have to keep following the rules and taking those simple steps that we know can save lives: staying at home whenever possible, and washing our hands, covering our face, making space and limiting social contact. Test, trace and isolate – including of those without symptoms – will remain at the heart of our approach, and we urge everyone to continue to cooperate with these vital efforts because every citizen has a responsibility to take the steps needed to control this virus.
Just as we have become accustomed to seeing grim statistics each day, we hope we will all enjoy watching the numbers go up and up in the weeks and months to come, safe in the knowledge that this time it means more vaccines are being given, more lives are being saved and we’re all moving towards a brighter future.
Matt Hancock, Secretary of State for Health and Social Care
Nadhim Zahawi, Parliamentary Under Secretary of State (Minister for Business and Industry) and Parliamentary Under Secretary of State (Minister for COVID Vaccine Deployment)
Executive summary and scope
Vaccines are a foundation of our way out of this pandemic and the best way to protect people from COVID-19, potentially saving thousands of lives. Since the emergence of COVID-19 there has been a global quest to find a vaccine. UK scientists were tracking the emergence of the new virus from early January 2020 and as soon as Chinese investigators released the genetic code for the virus, they began work to develop a vaccine to counteract it.
Back in 2016, the UK Vaccine Network (UKVN) provided funding to support Oxford University to develop a vaccine for Middle East Respiratory Syndrome (MERS). This vaccine technology was rapidly repurposed to develop a COVID-19 vaccine using initial funding from a National Institute for Health Research (NIHR) and UK Research and Innovation (UKRI). The UK should be proud of its history of research into vaccines and viruses, which have been publicly funded for nearly a century. Thanks to this historic funding and fast, ground-breaking research, to date, 3 vaccines have been approved by the Medicines and Healthcare products Regulatory Agency (MHRA), after meeting their strict standards of safety, quality, and effectiveness.
A successful vaccination programme will protect people from serious illness and help us all return to a more normal life.
The UK has a very successful record delivering vaccination programmes, but this is the biggest vaccination programme in NHS history. We have an ambitious timetable. By 15 February we aim to have offered a first vaccine dose to everyone in the top 4 priority groups identified by the Joint Committee on Vaccination and Immunisation (JCVI):
-
all residents in a care home for older adults and their carers
-
all those 80 years of age and over and frontline health and social care workers
-
all those 75 years of age and over
-
all those 70 years of age and over and clinically extremely vulnerable individuals
This plan describes how we were able to build up a supply of vaccines and how we are planning to deploy them. The plan has 4 key parts:
-
Supply
-
Prioritisation
-
Places
-
People
Co-operation across and between the 4 nations of the UK has been a key part of our approach to tackling the virus. The 4 UK Chief Medical Officers have taken a joint approach in their advice regarding the vaccine and securing supply of vaccines is a UK-wide effort. Each of the UK nations has plans to scale up vaccine delivery and the Places and People sections of this plan set out the deployment plans of the NHS in England. While exact models will differ slightly from region to region, all 4 nations’ plans involve a mixture of delivery models which include mobile teams to visit care homes, large sites at hospitals, and primary care-based delivery.
Supply
The need to invest in vaccines and develop them quickly in a pandemic was recognised by government well ahead of COVID-19. The UK government took concerted and coordinated action to invest £120m between 2016 and 2021 for the development of new vaccines, in line with the expert advice provided by the UKVN, made up of leading experts from academia, industry and policy. The UKVN funded Oxford University £1.87m to develop a MERS (another coronavirus) vaccine. This MERS vaccine technology was rapidly repurposed to develop a COVID-19 vaccine using initial funding from an NIHR and UKRI research call launched in February.
In April the government announced £20m of further funding so that the Oxford clinical trials could commence immediately.
The UK government’s Vaccine Taskforce (VTF) was established in April 2020 to ensure that the UK population would have access to a safe and effective vaccine against COVID-19. The VTF’s approach enabled different stages of the vaccine development process to take place quickly and in parallel, without ever compromising strict safety, quality and effectiveness standards. The MHRA have been constantly reviewing data from vaccine clinical trials, rather than waiting for delivery of all the data at the end of the process.
The VTF built a wide portfolio of different types of vaccines to give the UK the best possible chance of having a highly effective vaccine. The VTF focused on vaccines that could be available as soon as possible, which could be manufactured in large quantities (preferably in the UK) and could be delivered as rapidly as possible.
The VTF moved quickly to sign deals to buy the most promising vaccines and the UK has so far secured access to 367 million doses from 7 vaccine developers with 4 different vaccine types. The UK was the first country in the world to buy the Pfizer/BioNTech vaccine, ordering 40 million doses – enough for a third of the UK population – the first to authorise it and the first to begin vaccinating people with it. The UK was also the first country in the world to buy, authorise and provide the Oxford/AstraZeneca vaccine.
The VTF has rapidly expanded our vaccine manufacturing capability. ‘Fill and finish’ where vaccines are filled and sealed in multi dose vials is a critical stage in this process but one that has been under strain as a result of the pandemic, with a global shortage in fill and finish capacity. The VTF’s deal with Wockhardt in 2020 has given the UK its own substantial fill and finish capacity. This enabled the rapid increase in production of the Oxford/AstraZeneca vaccine at Wockhardt’s facility in North Wales.
Prioritisation
The Joint Committee on Vaccination and Immunisation (JCVI), advised that the priority for the current COVID-19 vaccination programme should be the prevention of COVID-19 mortality and the protection of health and social care staff and systems. The Committee advised that for both Pfizer/BioNTech and Oxford/AstraZeneca, the vaccine should first be given to residents in a care home for older adults and their carers, then to those over 80 years old as well as frontline health and social care workers, then to the rest of the population in order of age and clinical risk factors.
After studying all the available data, the JCVI concluded that the first dose of either Pfizer/BioNTech or Oxford/AstraZeneca vaccine provides substantial protection within 2 to 3 weeks of vaccination from severe COVID-19 disease. The second vaccine dose is important to sustain the protection and extend its duration. In the short term, the additional impact of the second dose is likely to be modest and most of the initial protection from clinical disease is after the first dose of vaccine.
The 4 UK Chief Medical Officers agreed with the JCVI that at this stage of the pandemic prioritising the first doses of vaccine for as many people as possible on the priority list would protect the greatest number of at-risk people in the shortest possible time. Operationally this means that second doses of both vaccines will be administered towards the end of the recommended vaccine dosing schedule of 12 weeks. This will maximise the number of people getting the vaccine and receiving protection within the next 12 weeks.
Places
Our top priority is to offer a COVID-19 vaccine to everyone in JCVI cohorts 1 to 4 by 15 February. The network of vaccination sites has been designed to fit the expected vaccine supply and ensure safe and easy access for the whole population.
There are 3 types of vaccination site:
-
vaccination centres, using large-scale venues, such as football stadiums and accessed by a national booking service
-
hospital hubs, using NHS trusts across the country
-
local vaccination services, made up of sites led by general practice teams working together in already established primary care networks and pharmacy teams through community pharmacies
This mix will allow people in different age groups, communities and households to get a vaccine in a way that suits them and their needs.
The growing network of vaccination sites will rapidly expand in the days and weeks ahead. Currently, 96% of the population in England is within 10 miles of a vaccine service. By the end of January, everyone will live within 10 miles of a vaccination centre. In a small number of highly rural areas, the vaccination centre will be a mobile unit.
In England, by the end of January, our capacity to vaccinate several hundred thousand a day, and at least 2 million people per week will be achieved by establishing:
-
206 active hospital hub sites
-
around 1,200 local vaccination service sites (including primary care networks, community pharmacy sites, and including the ability to travel to those who cannot come to a centre)
-
50 vaccination centres
The scale of this challenge is enormous, equivalent to establishing a national supermarket business in less than a month. All parts of the healthcare system will be mobilised so that we can vaccinate the highest risk individuals as rapidly as possible. The network will continue to expand and evolve as we progress the deployment in the months ahead. We will expand the programme so all adults can be vaccinated by the autumn.
We know that the vast majority of people want to receive a vaccine but we need to ensure that our approach to deployment is inclusive and helps tackle inequalities by addressing individual concerns of those who are more hesitant or have questions about how the vaccine fits their particular circumstances, such as a medical condition, their age or ethnic background.
At national, regional and local level we are working in partnership with local authorities, the voluntary and community sector, local resilience forum, communities, staff and patients to ensure that simple accessible advice and information is available to everyone who needs it and that local implementation plans are tailored to support uptake in all communities. We are committed to ensuring that local authorities and directors of public health have the data they need to understand uptake in their local areas and tailor efforts to reach those who have not yet taken up the offer of a vaccine appointment.
We will provide advice and information at every possible opportunity to support those who have been prioritised to receive a vaccine and anyone who has questions about the vaccination process. The NHS website includes information on how you will be invited and what happens next and the process from invitation to follow-up is set out in the how you will be contacted and what happens next section of this plan. We want as many people as possible take up the offer of a COVID-19 vaccination when it is their turn.
People
The heart of the programme is its workforce. Thousands of people have joined the vaccination effort and we have recruited an 80,000 strong workforce ready to be deployed across the country. As well as our current NHS staff, this includes drawing on the skills of those who have volunteered through the NHS Bring Back scheme, those currently working outside of the NHS such as St John’s Ambulance and independent nurses and occupational health service providers. There are many other people in the UK who have vital experience and skills that can provide support like airline cabin crew. There are similar schemes across the devolved administrations.
As well as trained vaccinators, the COVID-19 vaccination programme will include a range of non-clinical support staff to ensure quick and easy access to a vaccine. For example, administration support, logistics, stewards and first aiders, as well as those who can log, record and manage stocks. Drawing on the logistical expertise of the Armed Forces, we are using tried and tested operational techniques.
In total, over 200,000 people have expressed their interest in playing their part. We want to thank everyone who has put themselves forward. The vaccination programme is a marathon not a sprint and not everyone who has volunteered will be deployed immediately, but we are working hard to ensure that people are prepared and informed, so that they can be mobilised as and when required.
Tracking our progress
The vaccination programme is committed to publishing clear and simple updates. Since 24 December, we have published weekly UK-wide data on the total number of vaccinations, and the breakdown between over and under 80s for England. From 11 January, daily data for England will be published showing the total number vaccinated to date, including first and second doses. On 14 January and then on a weekly basis, NHSEI will publish a more detailed breakdown of vaccinations in England, including by region.
Supply
Developing new vaccines
Vaccine development and delivery is a complex process that requires collaboration from experts across multiple disciplines, with no guarantee of success. Vaccine candidates typically have a high rate of failure during their clinical trials, and no vaccines have yet been developed for some diseases such as HIV.
UK scientists were tracking the emergence of the new virus from early January 2020 and as soon as Chinese investigators released the genetic code for the virus they began work to develop a vaccine to counteract it. The rapidity of the Oxford/AstraZeneca vaccine development was made possible by earlier research by the Oxford team on other pandemic prone pathogens, including the MERS coronavirus, supported by the UK Vaccines Network since 2016. This technology was repurposed using funding from the rapid research call on COVID-19 launched in February 2020 and funded by the NIHR and UKRI. NIHR has provided support for 5 COVID-19 vaccine trials to date and used its established network of trial sites within the NHS. The NIHR, working with industry and government partners, is widely recognised as having transformed the environment for clinical research and trials in the UK, and this has made the UK a top choice for companies doing COVID-19 vaccine research. The UK should be proud of its history of research into vaccines and viruses publicly funded for nearly a century.
In January 2020, the Medical Research Council, part of UKRI funded £0.5m each to 2 centres:
- the MRC Centre for Global Infectious Disease Analysis at Imperial College London, which has been contributing world-leading outbreak modelling and transmission dynamics and liaising with WHO
- the MRC-University of Glasgow Centre for Virus Research, which has investigated the genetics and origins of the virus
The NIHR funded £47.5m between 2014 and 2020 in 13 Health Protection Research Units (HPRUs) to research high-priority areas in public health. This included work from the HPRU in Emerging and Zoonotic Infections to allow PHE to rapidly assess the pandemic potential and risk posed by new and emerging infections (particularly zoonotics) and work from the HPRU in Respiratory Infections on surveillance, modelling and vaccine research to ensure a rapid public health response to new and emerging threats.
In March 2020, the government, including NIHR and UKRI, invested £20m in the COVID-19 Genomics UK Consortium to deliver large scale, rapid sequencing of the cause of the disease and share intelligence with hospitals, regional NHS centres and the government. The UK consortium, which includes the NHS, PHE, UKRI, and the Wellcome Sanger Institute, enables clinicians and public health teams to rapidly investigate clusters of cases in hospitals, care homes and the community, to understand how the virus is spread and implement appropriate infection control measures. It has recently been in the lead in identifying and characterising the spread, and impacts, of new variants of the virus.
The UK government’s Vaccine Taskforce (VTF) was established in April 2020 by the government’s Chief Scientific Advisor, Sir Patrick Vallance, to drive forward, expedite and co-ordinate efforts to ensure that the UK population would have access to a clinically safe and effective vaccine against COVID-19. Kate Bingham was appointed in May 2020 as Chair of the VTF reporting directly to the Prime Minister and working within BEIS. She was succeeded by Clive Dix as interim Chair in December 2020.
The Prime Minister asked the VTF to deliver 3 objectives:
-
Secure access to promising COVID-19 vaccine/s for the UK population as quickly as possible
-
Make provision for international distribution of vaccines
-
Support the UK’s industrial strategy by establishing a long-term vaccine strategy and to prepare the UK for future pandemics
Since it was established, the VTF has successfully built a portfolio of vaccine candidates that includes established vaccine platforms and newer but clinically advanced, state-of-the-art platforms. The UK’s vaccine portfolio is diversified across 4 different vaccine technologies to maximise the chances of finding a successful vaccine.
The UK has so far secured access to 367 million doses from 7 vaccine developers across 4 different formats (viral vectored vaccines, recombinant protein-based adjuvanted vaccines, whole inactivated viral vaccines and mRNA vaccines), with an expected cost of £2.9bn across the five final contracts signed to date. As announced in the Spending Review, the government has now made available more than £6bn in total to develop and procure COVID-19 vaccines.
Table 1: portfolio overview
| Vaccine type | Vaccine | No of doses | Status |
|---|---|---|---|
| Adenovirus | Oxford/AstraZeneca | 100 million | Approved and in deployment |
| Adenovirus | Janssen | 30 million | Phase 3 trials |
| mRNA | Pfizer/BioNTech | 40 million | Approved and in deployment |
| mRNA | Moderna | 17 million | Approved |
| Protein Adjuvant | GlaxoSmithKline/Sanofi Pasteur | 60 million | Phase 1/2 trials |
| Protein Adjuvant | Novavax | 60 million | Phase 3 trials |
| Inactivated whole virus | Valneva | 60 million | Phase 1/2 trials |
Global leadership
The Prime Minister has committed the UK to work for equitable global access to COVID-19 vaccines. We have been instrumental in establishing COVAX – an international initiative to support the discovery, manufacture and fair distribution of vaccines for both lower and higher income countries. COVAX aims to procure vaccine for one billion people in some 180 countries by the end of 2021. The UK is one of the largest donors.
We have committed £548m for vaccines for lower income countries and through matched funding mobilised other countries to raise over $1bn for the COVAX Advanced Market Commitment (AMC). At the United Nations General Assembly in September, the UK announced that it would match every $4 pledged to the COVAX AMC by other donors with £1 in UK funding, up to £250m. Since then, other countries including Canada, Japan and Germany have committed funding to the scheme, combining to reach the landmark target of $1bn.
We have also put in a further £71m to secure rights to purchase up to 27 million doses for the UK population if needed. We are focusing on supporting COVAX to deliver quickly and to develop a mechanism to enable countries with surplus doses to distribute them equitably. The UK will use its presidency of the G7 this year to further mobilise world leaders to support global efforts to end this pandemic and to seize the moment to strengthen international readiness for pandemics in line with the five-point plan that the Prime Minister set out at the UN General Assembly in September.
The UK government has been strongly supportive of the aims of the Coalition for Epidemic Preparedness Innovations (CEPI) since its inception in 2016, contributing £260m to support CEPI’s work on vaccine development, including against COVID-19. CEPI supported vaccines include Moderna and Novavax.
The MHRA is continuing its significant international effort working with regulators around the world on matters relating to COVID-19 vaccines. MHRA is playing an active role in the International Coalition of Medicines Regulatory Authorities (ICMRA) where we co-chair the COVID-19 Working Group, lead a project on the digital transformation of inspections and take part in regular pharmacovigilance discussions.
We are working together with the Access Consortium (Australia, Canada, Switzerland, Singapore and the UK) on regular information sharing with regards to ongoing assessments but also on post-market surveillance, where we are working to high standards of scientific rigour and integrity, with reduced regulatory duplication.
Accelerated timetable
The timetables for developing COVID-19 vaccines have been accelerated significantly, thanks to the efforts of all parties involved, without compromising on safety, quality and effectiveness. For example, developers have run trials concurrently; the government has provided funding for manufacturing “at risk”, ahead of vaccines receiving regulatory approval; and the MHRA has been reviewing efficacy data on a rolling basis, rather than waiting for all of the data to become available at once.
Figure 1: traditional vaccine development timeline – several years
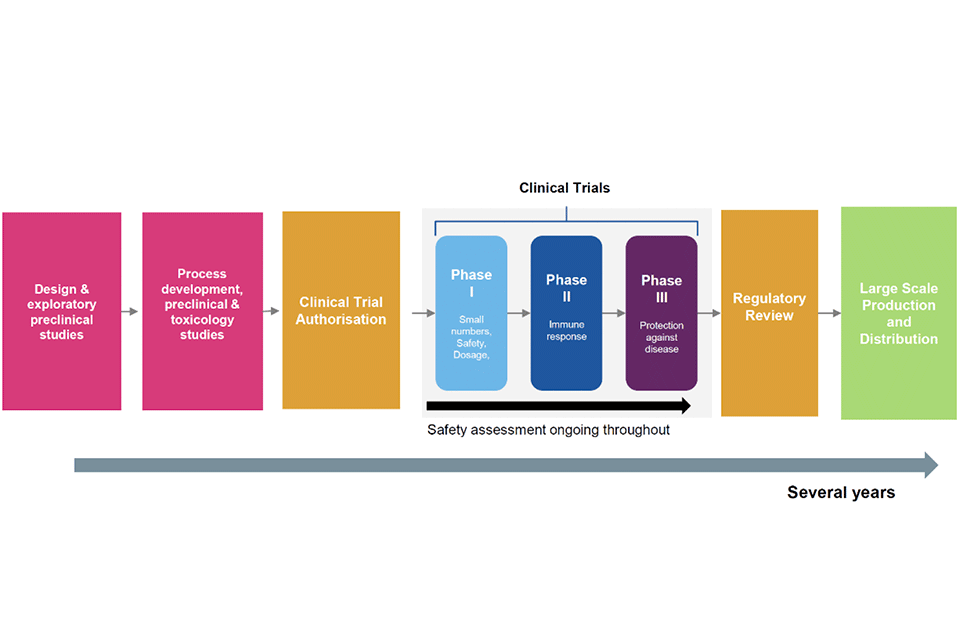
The figure shows the traditional vaccine development that takes several years. Beginning with design and exploratory preclinical studies; followed by process development, preclinical and toxicology studies; clinical trial authorisation, phase 2 to 3 clinical trials; regulatory review; and finally large scale production and distribution.
Figure 2: COVID-19 accelerated timeline – 10 months plus
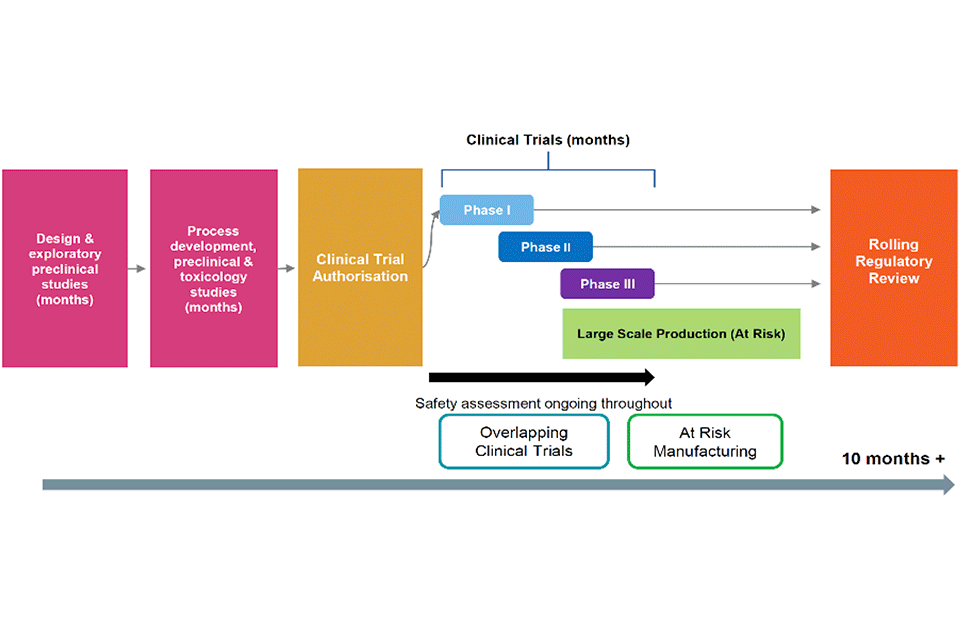
The figure shows the accelerated vaccine development timeline that has taken 10 months plus. Beginning with design and exploratory preclinical studies; followed by process development, preclinical and toxicology studies; and clinical trial authorisation. Safety assessment is ongoing throughout through overlapping clinical trials and at risk manufacturing. Regulatory review is done on a rolling basis.
The VTF’s strategy was to move quickly to strike deals with the most promising vaccine candidates. This approach, together with the efforts made by suppliers, has offered several advantages to the UK and ultimately enabled the rapid availability of COVID-19 vaccines in the UK. The UK was the first country in the world to secure access to the Pfizer/BioNTech vaccine and to start deploying it as an authorised vaccine (under Regulation 174). The UK was also the first country to procure, authorise (under Regulation 174) and commence deployment of the Oxford/AstraZeneca vaccine.
Striking early deals at risk has also provided additional time to prepare for deployment. This additional time has been particularly important where vaccines are challenging to deploy – for example, if they have particular storage requirements such as low temperature storage – and has helped minimise the time between MHRA authorisation and the start of vaccine deployment for both the Pfizer/BioNTech and Oxford/AstraZeneca vaccines.
A Great British success story: the Oxford University/AstraZeneca vaccine
The UK is the first country in the world to begin rollout of the adenovirus vectored vaccine that has been developed by the Jenner Institute and Oxford University Group at Oxford University, in partnership with AstraZeneca.
In 2016, the UK Vaccine Network provided funding to support Oxford University to develop a vaccine for MERS. This was part of a wider initiative to invest in vaccines for emerging diseases that cause outbreaks in low- and middle-income countries and have the potential to cause future epidemics or pandemics. This vaccine technology was rapidly repurposed to develop a COVID-19 vaccine using initial funding from a NIHR and UKRI research call launched in February.
UK scientists were tracking the emergence of the new virus from early January 2020 and as soon as Chinese investigators released the genetic code for the virus, they began work to develop a vaccine to counteract it. As a result, the Oxford vaccine candidate developed very quickly from an academic idea developed by Professor Sarah Gilbert with her colleague Professor Teresa Lambe at the Jenner Institute into a world-leading COVID-19 vaccine candidate. A trial of over 23,000 people around the world, led by Professor Andy Pollard at the Oxford Vaccine Group, and supported by the NIHR Clinical Research Network and equivalents in the devolved administrations, has produced positive safety and efficacy data. Oxford University and the Jenner Institute were able to reach an early agreement with AstraZeneca for them to operate the global development, manufacture and distribution of the vaccine.
The Oxford University/AstraZeneca vaccine, which is being produced on a not-for-profit basis for the period of the pandemic, and in perpetuity in low- and middle-income countries, can be stored at fridge temperature, making it easy to transport and distribute, not just across the UK, but around the world, and will be a key tool in the global fight against the virus.
Just under a year since COVID-19 research began, doses of the authorised vaccine manufactured at production sites in Oxfordshire and Staffordshire, and fill and finished in North Wales, are already being deployed to protect thousands of people in the UK every day against the virus.
The rapidity of the Oxford/AstraZeneca vaccine development was made possible by earlier research by the Oxford team on other pandemics, including the MERS coronavirus. The UK should be proud of its history of research into vaccines and viruses, which have been publicly funded for nearly a century. The NIHR, working with industry and government partners, is widely recognised as having transformed the environment for clinical research and trials in the UK. Early in the pandemic the NIHR put in place a system for prioritising research, which supported the most important studies to recruit, which included the Oxford vaccine studies.
The government has continued to work closely with Oxford University to ensure enough funding to complete vital clinical trials, using the NIHR networks and prioritisation process and to accelerate manufacturing. To date the government has announced over £88m in funding to help optimise the development process, scale up manufacturing and enable the vaccine to be made available as soon as possible to the British public.
The VTF focused on vaccines that could be available as soon as possible, could be manufactured at scale (preferably in the UK), had the potential to secure rapid regulatory approval and could be delivered ready for deployment as rapidly as possible.
This approach has proven successful: just 9 months after the VTF’s establishment, promising safety and efficacy data for 3 of the 7 vaccines in the VTF’s portfolio has been released, three have already been authorised and 2 have begun to be rolled out across the UK. At time of publication, the health services have already vaccinated over 2 million people in the UK.
Looking forward, the UK’s vaccine supply and scheduled deliveries will fully support vaccination of JCVI priority cohorts 1 to 4 by 15 February. The government has signed deals for substantial future supply of both the Pfizer/BioNTech and Oxford/AstraZeneca vaccines to replenish the UK’s stocks and enable swift vaccination of first and second doses across the UK in the weeks and months ahead. The government has also ordered an additional 10 million doses of the Moderna vaccine, taking its total to 17 million.
National vaccines research citizen registry
The Vaccine Taskforce has also worked closely with the NIHR and NHS Digital to develop and launch the world’s first national citizen registry. Hosted on the NHS website, the registry allows the British public to sign up and register their interest in being contacted about vaccine trials taking place across the UK. At the time of writing, over 367,000 people have registered from a diverse range of backgrounds, which is vital to ensure potential vaccine candidates work for all. The National Institute for Health Research is supporting 5 vaccine clinical trials in the UK:
- Oxford/AstraZeneca
- Imperial College London
- Novavax
- Valneva
- Janssen
Over 120,000 people have been contacted by the registry about taking part in a vaccine clinical trial. This, and the NIHR prioritisation process for research, has enabled rapid recruitment into 5 different UK clinical vaccine trials ensuring they can progress without delay to generate the necessary safety and clinical efficacy data. Under the accelerated development timeline developers have been running overlapping clinical trials, reducing the time delay between each trial. The MHRA has also worked to expedite vaccine approvals, as well as using a pre-assessment process with researchers throughout trials, from the time of initiation and as they progress. Rigorous safety and quality standards are applied as for any clinical trial requiring MHRA approval, however the pre-assessment and expedited reviews allowed for approval of the Oxford/AstraZeneca vaccine trials within 7 working days.
Case study: Human Challenge Programme
The government has also invested in developing a new clinical trial capability to accelerate vaccine development using human challenge studies. The Human Challenge Programme will carry out controlled testing of new vaccines, where young healthy volunteers will receive the vaccine prior to being infected with the virus.
Human challenge studies have been safely carried out for many years and have played important roles in developing vaccines for diseases including typhoid, and flu. The government’s Human Challenge Programme will likely be the first such study in COVID-19 in the world. The UK is a leader in human challenge studies and has the infrastructure and skilled workforce to deliver this programme, which will develop our long-term research capability in human challenge studies, ready for future pandemics.
Ensuring vaccines meet strict safety standards for deployment
As with any medicine, vaccines are highly regulated products. A COVID-19 vaccine will only be authorised for use once it has met strict standards of safety, effectiveness and quality through clinical trials and data assessments. The MHRA is the UK’s regulator whose role is to ensure medicines, devices and vaccines work effectively and are safe for use. There are checks at every stage in the development and manufacturing process and each COVID-19 vaccine candidate is assessed on a case by case basis.
The data reviewed includes all the results from laboratory studies, clinical trials, manufacturing and quality controls and testing the product. Only once these tests have demonstrated that the vaccine meets the required standards of safety, quality and efficacy can a COVID-19 vaccine been made available
Teams of scientists and clinicians carefully, methodically, scientifically and rigorously review all data on safety, effectiveness and quality as soon as they become available, and have done so throughout all tests and trials.
Vigilance and Surveillance
Authorised COVID-19 vaccines are monitored continuously after roll-out by the MHRA and PHE to ensure that the vaccines perform as expected in routine use and for all population groups. This will help to confirm that the benefit of the vaccines continues to outweigh any risk.
The MHRA has statutory responsibility for continuous safety monitoring following vaccine approval. The MHRA has 4 elements to their real-time pharmacovigilance strategy in place for COVID-19 vaccines, guided by a dedicated Expert Working Group of the Commission on Human Medicines.
Suspected side effects to COVID-19 vaccines can be reported through the Coronavirus Yellow Card reporting portal. The MHRA are proactively encouraging reports through a targeted communications campaign.
Proactive surveillance to follow up selected groups who may have been under-represented in clinical trials to further investigate the safety of the vaccine in these populations.
Use of electronic health records to monitor adverse events of special interest – including events that could theoretically be related to vaccinations. This Rapid Cycle Analysis will analyse recorded post-vaccination incidence rates against historical comparator groups and will produce a continuous read-out of cases to identify if we are seeing more cases in a period following vaccination than we would expect naturally.
Epidemiology studies using electronic health record data – ad hoc analyses to quickly identify changing trends in rates of illness at ‘population’ level and to test important hypotheses (safety signals) that may arise from the above surveillance or other sources (for example in other countries).
The MHRA are working in collaboration with partners in the health system to rapidly assess all available safety data in real time and communicate any emerging issues, as necessary. To provide reassurance in the ongoing benefit and risk assessment of available vaccines; the MHRA will publish details of all suspected reactions reported in association with available COVID-19 vaccines, along with our assessment of the data on a regular basis.
PHE has responsibility for surveillance of the COVID-19 vaccination programme and has developed a surveillance strategy (published in parallel on 12 January 2021) encompassing monitoring of vaccine coverage, symptomatic disease, asymptomatic infection, and seroprevalence (the prevalence of immunity in the population). Working with the NHS and academic centres these detailed plans will allow us to document whether the vaccine interrupts transmission, how the vaccine works in people with underlying conditions, how long protection lasts and whether the changes in the circulating virus affect the protection received from the vaccine.
Because we continue to test many of those who have received the vaccine – including NHS and social care staff – we will be able to monitor very closely the real-world impact of the vaccination on those vaccinated early in the programme.
The PHE surveillance strategy includes a plan to monitor vaccine coverage in the general population using 2 parallel approaches:
-
a weekly aggregated data extract from GP electronic health records via the ImmForm platform, as used for existing national immunisation programmes such as seasonal influenza. This allows comparison of coverage in key clinical risk groups, as it is linked to patients’ clinical information
-
a weekly extract from the new National Immunisation Management System (NIMS) commissioned by NHSEI. As well as providing rapid information for monitoring the pace of delivery, this provides a central register of vaccines delivered in the full range of health care settings.
Investigating any impact of the new variant
As part of our ongoing monitoring, we want to assess any effect the new variant (variant B1.1.7) of COVID-19 may have on the vaccine. The SIREN (SARS-CoV-2 Immunity & Reinfection Evaluation) is a major ongoing study testing blood samples and swabs obtained from healthcare workers who work in a clinical setting. 228 trusts in England, 6 in Northern Ireland and 15 in Scotland are currently participating in SIREN. As part of the study, healthcare workers are being tested every 4 weeks via a venous blood text, every 2 weeks via polymerase chain reaction (PCR) screening, and twice weekly lateral flow devices.
In response to the new variant of COVID-19, we are building upon and boosting this existing study to understand any effect the variant B1.1.7 may have on the vaccine’s effectiveness. This includes a targeted recruitment drive for healthcare workers in parts of the country with a higher risk of catching variant B1.1.7, including London, Essex and Kent. NIHR and the CMO have asked NHS trusts’ research teams to prioritise and offer participation to the SIREN study to all healthcare workers who are receiving COVID vaccines. PHE has updated the study’s protocol and received ethics approval for amendments.
If, alongside your vaccination, you are invited to participate in this study, you are strongly encouraged to take part. Initial analysis will be conducted at the end of January, but further phases of analysis may be required depending on number of individuals recruited.
Our exceptional network of manufacturers and academics are already responding to the new variants that have been identified and we will continue to draw on and support private sector technical and manufacturing expertise to ensure a rapid response if a variant does emerge that means the current vaccines are less effective. If this happens, our aim is that, as soon as scientifically possible, the UK will have continued access to effective vaccines.
Building UK manufacturing capability
In parallel to securing access to promising vaccine candidates, the VTF has worked rapidly to expand the country’s manufacturing capabilities, both to ensure that the UK was in a position to manufacture millions of doses of a successful COVID-19 vaccine (if required) and to develop that capacity in response to any potential future pandemics.
Manufacturing vaccines
There are several processes involved in the manufacture of a vaccine. Broadly these are:
-
trial batches: to check the process and adjust to safely produce a consistent drug substance to specified requirements. This must be carried out at every plant prior to production commencing
-
manufacture: to produce bulk drug substance
-
fill and finish set up: before each new product introduced, the equipment must be cleaned, and trial batches produced and tested to demonstrate its safety for the drug substance production
-
fill and finish: to fill and seal in multi dose vials. Such vials will be marked with identification of product, production batch reference, expiry date, shelf life and storage conditions
-
testing: the product is sampled and tested throughout the manufacturing and filling process for consistency and microbiological integrity
-
final packaging: to pack and carry out final testing to ensure compliant product is sent to users. Such final packs will again display product description, batch number and expiry date
The government has provided funding in several UK sites to secure rapid manufacturing capability of millions of doses of vaccine, with the flexibility to manufacture different platforms. This allows the UK to maintain optionality in manufacturing capability in the longer term.
Three of the 7 candidates in the VTF vaccine portfolio are being manufactured in the UK:
- AstraZeneca’s adenoviral vaccine
- Valneva’s whole virus vaccine
- Novavax’s VLP protein adjuvant vaccine
As well as the obvious logistical benefit of manufacturing in the UK, we have a thriving life sciences sector and clinical research infrastructure in the UK as the partnership between Oxford and AstraZeneca illustrates
Investing in vaccine manufacturing
The government’s manufacturing investments and support include:
-
the acceleration and expansion of both drug substance and fill and finish manufacturing capabilities at the UK’s first Vaccine Manufacturing and Innovation Centre (VMIC) at Harwell, building on the previous investment made through UKRI. VMIC is the UK’s first facility dedicated to mass manufacturing vaccines. It will develop novel innovative manufacturing processes and carry out vaccine manufacturing at scale
-
the establishment of a rapid deployment facility at Oxford Biomedica which will manufacture the Oxford/AstraZeneca vaccine drug substance at scale, and early support to develop manufacturing skills
-
collaborating with the Cell and Gene Therapy Catapult to fund a state-of-the-art facility in Braintree
-
supporting the development of Valneva’s manufacturing facility in Livingston, Scotland to support scale up, creating a major UK vaccine facility capable of mass-producing vaccines
-
partnering with Wockhardt to provide fill and finish services as part of the effort to accelerate vaccine manufacturing in the UK.
Fill and finish
As a result of the pandemic, there is a global shortage in fill and finish capacity. This stage of the manufacturing process is critical to our ability to roll out a successful vaccine in deployable doses. Identifying limited global supply of fill and finish capacity as a potential bottleneck in the manufacturing process, the VTF entered into an agreement with Wockhardt in 2020 to secure fill and finish capacity for the UK over the coming year, including for the Oxford Astra/Zeneca vaccine which is currently being fill and finished at Wockhardt’s facility in North Wales.
Wockhardt is a global pharmaceutical and biotech organisation that brings affordable, high quality medicines to market. In the UK, Wockhardt is one of the largest suppliers of medicines including insulin and diamorphine into the NHS for over 20 years. It has had a presence in Wrexham for over 2 decades and employs over 400 people at its 612,000 square feet high-tech manufacturing facility.
Wockhardt UK is known for its manufacture and supply of generic and branded medicines, with an expanding portfolio of over 250 products.
Expanding our skilled workforce to support manufacturing capabilities
Investment in upskilling our workforce, technology transfer capability and innovation will boost the UK’s attractiveness as a manufacturing centre for vaccines and related advanced therapies. This will also help maintain UK readiness and resilience in the event of future pandemics and ensure that the UK stays at the forefront of global vaccine research and development.
The Vaccines Taskforce have funded the Cell and Gene Therapy Catapult with £4.7m to develop the Advanced Therapy Skills Training Network (ATSTN) in collaboration with industry, to help grow the sector by creating economic opportunities for new jobs and industry-driven learning. With the industry workforce expected to double to more than 6,000 by 2024, this investment is continuing the government’s commitment to expanding the UK expertise in advanced therapies, building on previous successful skills and career programmes with the industry. This is a significant contribution to ensuring the continuous growth of the UK advanced therapies and vaccine manufacturing industry, developing the nation’s health resilience by creating opportunities for current and new skilled personnel.
There are 3 different components to the ATSTN:
- online training platform
- a coordinated network of national training centres
- an accessible online career converter
There are many opportunities in this growing sector and the cutting-edge ATSTN capabilities will facilitate attracting new skills from other sectors, plus accelerating the skills growth within the sector.
Regulatory Approval and Batch Testing
Once a vaccine has produced enough data to demonstrate its safety, efficacy, and quality, the UK has 2 regulatory routes open to authorise it for deployment.
The first of these is for the government to issue a temporary authorisation under Regulation 174 of the Human Medicines Regulations 2012 (which were amended in 2020 to allow for this to happen) based on the MHRA’s analysis and recommendations on safety, efficacy and quality. This permits the deployment of unlicensed medicines, on a temporary and emergency basis, to meet certain specified public health needs, such as those arising from a pandemic.
The second, more routine option is for the MHRA to issue a marketing authorisation (also called a product license) based on their assessment of the vaccine’s safety, efficacy and quality. Until 31 December 2020, the power to grant marketing authorisations for products such as vaccines in respect of the UK was reserved solely to the EU regulator, the EMA. Since 1 January 2021, the MHRA have been able to issue such licenses themselves in Great Britain.
Figure 3: regulatory process flow for COVID-19 vaccines
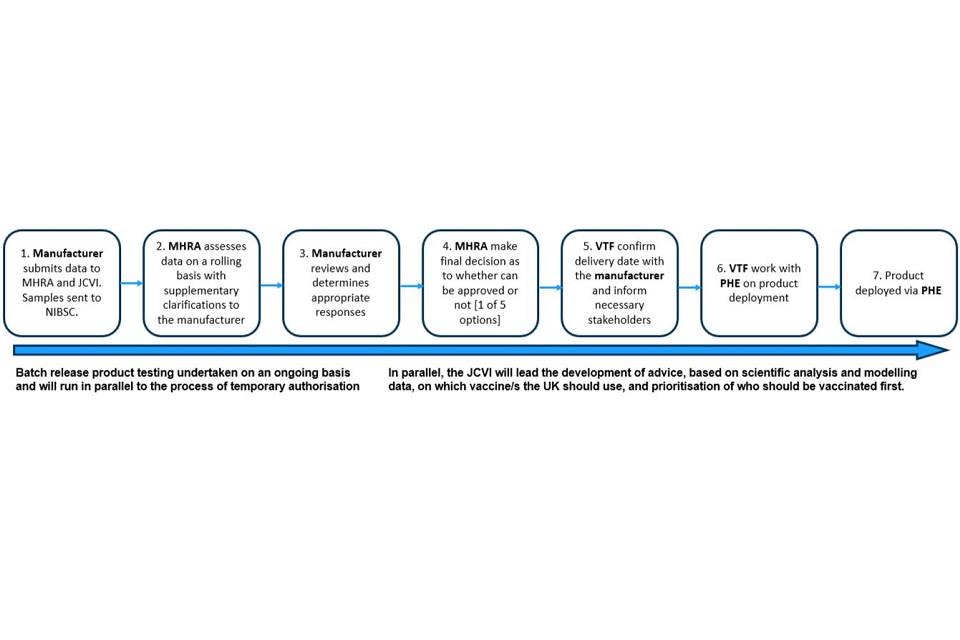
This image shows the steps in the regulatory process for COVID-19 vaccines. First, the manufacture submits data to MHRA and JCVI. Samples are sent to NIBSC. Secondly, MHRA assesses data on a rolling basis with supplementary clarifications to the manufacturer. Thirdly, manufacturer reviews and determines appropriate responses. Fourth, MHRA make final decisions as to whether can be approved or not. Fifth, VTF confirm delivery date with the manufacturer and inform necessary stakeholders. Sixth, VTF work with PHE on product deployment. Finally, the product is deployed to PHE or other national organisation for deployment.
For both regulatory routes the MHRA have utilised flexibilities, such as rolling review, to ensure that decision-making is as fast as is feasible without compromising the UK’s high standards of safety, efficacy or quality. These flexibilities have allowed the UK to authorise use of Pfizer, Oxford/AstraZeneca, and Moderna’s vaccines within weeks of Phase 3 safety and efficacy data becoming available.
In addition to securing the appropriate regulatory approvals, all vaccines must undergo rigorous batch testing by the manufacturer and independent batch testing by the MHRA before deployment. The vaccines are complex biological products and the manufacturer’s testing needs to comply with rigorous international standards. To increase speed the MHRA independent batch release testing is done in parallel with the testing by the manufacturer. Once the MHRA receive the manufacturer’s own completed testing data, it typically takes less than 24 hours to both complete the final independent review and, provided everything meets the product specification, to issue a certificate confirming the batch as being of suitable quality for use. This testing is done throughout the whole manufacturing period and prior to release of each batch for deployment. In the case of both the Pfizer and Oxford AstraZeneca vaccines, batch testing of the initial production batches was carried out in parallel with the regulatory approval process to expedite deployment.
Prioritisation
Prioritising the vaccine for those most at risk
The Joint Committee on Vaccination and Immunisation (JCVI) is the independent medical and scientific expert body which advises the UK government on prioritisation for all vaccines. JCVI have advised that the first priorities for the current COVID-19 vaccination programme should be the prevention of COVID-19 mortality and the protection of health and social care staff and systems.
Phase 1
For phase 1, the committee have advised that for both Pfizer/BioNTech and Oxford/AstraZeneca, the vaccine should first be given to residents in a care home for older adults and their carers, then to those over 80 years old as well as frontline health and social care workers, then to the rest of the at-risk population in order of age and clinical risk factors. These are set out in 9 cohorts.
The government’s top priority is to ensure that everyone in cohorts 1 to 4 is offered the opportunity to receive their first dose of vaccination against COVID-19 by 15 February. It will likely take until spring to offer the first dose of vaccination to the JCVI priority groups 1 to 9, with estimated cover of around 27 million people in England and 32 million people across the UK.
It is estimated that taken together, these at-risk groups account for 99% of all deaths from COVID-19 to date.[footnote 1] It is acknowledged that people can move into a higher group at any time, for example if their medical condition or age bracket changes. When this happens, they will be brought into the process as soon as possible.
Table 2: number of people in each cohort for vaccination under JCVI priorities[footnote 2]
| JCVI cohort | Priority group | England | UK | % deaths attributed to cohorts |
|---|---|---|---|---|
| Care home residents | 1 | 0.3m | 0.3m | - |
| Residential care workers | 1 | 0.4m | 0.5m | - |
| 80+ | 2 | 2.8m | 3.3m | - |
| Healthcare Workers | 2 | 2.0m | 2.4m | - |
| Social Care Workers | 2 | 1.2m | 1.4m | - |
| 75-79 | 3 | 1.9m | 2.3m | - |
| 70-74 | 4 | 2.7m | 3.2m | - |
| Clinically Extremely Vulnerable (under 70) | 4 | 1.0m | 1.2m | - |
| Total priority cohorts 1 to 4 | - | ~12m | ~15m | 88% |
| 65-69 | 5 | 2.4m | 2.9m | - |
| At Risk (under 65) | 6 | 6.1m | 7.3m | - |
| 60-64 | 7 | 1.5m | 1.8m | - |
| 55-59 | 8 | 2.0m | 2.4m | - |
| 50-54 | 9 | 2.3m | 2.8m | - |
| Total priority cohorts 5 to 9 | - | ~14m | ~17m | 11% |
| Total priority group population | - | ~27m | ~32m | 99% |
| Rest of adult population | - | ~18m | ~21m | - |
| Total | - | ~44m | ~53m | - |
JCVI advised that the implementation of the COVID-19 vaccine programme should aim to achieve high vaccine uptake. While the programme seeks to achieve 100% coverage for all groups, best practice in existing programmes has achieved 75% of total population cohorts. An age-based programme will likely result in faster delivery and better uptake in those at the highest risk. Within the guide set out by the JCVI framework, implementation should also involve flexibility in vaccine deployment at a local level with due attention to:
-
mitigating health inequalities
-
vaccine product storage, transport and administration constraints
-
exceptional individualised circumstances
Dosing strategy for Phase 1: protecting the greatest number of at-risk people in the shortest possible time
Underpinned by publicly available scientific data (set out in the annex: scientific evidence underpinning decisions on vaccination programme, the JCVI announced on 30 December 2020 that the first dose of either Pfizer/BioNTech or Oxford/AstraZeneca vaccine provides substantial protection within 2 to 3 weeks of vaccination for clinical disease, and in particular in preventing severe COVID-19 disease. The second vaccine dose is likely to be very important for duration and sustaining such protection, and at an appropriate dose interval may further increase vaccine efficacy. In the short term, the additional impact of the second dose is likely to be modest and most of the initial protection from clinical disease is after the first dose of vaccine.
In their letter to the profession, the 4 UK Chief Medical Officers agreed with JCVI that at this stage of the pandemic prioritising the first doses of vaccine for as many people as possible on the priority list will protect the greatest number of at risk people overall in the shortest possible time and will have the greatest impact on reducing mortality, severe disease and hospitalisations and in protecting the NHS and equivalent health services.
As set out by the 4 CMOs, for every 1,000 people boosted with a second dose of COVID-19 vaccine (who will as a result gain marginally on protection from severe disease), 1,000 new people do not benefit from substantial initial protection which is in most cases likely to raise them from 0% protected to at least 70% protected. These unvaccinated people are far more likely to end up severely ill, hospitalised or in some cases dying without vaccine. Halving the number vaccinated over the next 2 to 3 months because of giving 2 vaccines in quick succession rather than with a delay of 12 weeks does not provide optimal public health impact. Operationally this means that second doses of both vaccines will be administered towards the end of the JCVI recommended vaccine dosing schedule of 12 weeks. This will maximise the number of people getting the vaccine and therefore receiving protection within the next 12 weeks.
Phase 2: achieving protection of the whole UK population from COVID-19
As the first phase of the programme is rolled out across the UK, the government will consider all relevant data and set out plans for Phase 2 of vaccination once all at-risk groups 1 to 9 have been offered their first dose of vaccine. Phase 2 of the roll-out may include further reduction in hospitalisation and targeted vaccination of those at high risk of exposure and/or those delivering key public services.
The role a vaccine can play in supporting the resumption of normal life depends on how the vaccine works – whether the vaccine primarily acts by preventing symptomatic disease including severe disease, or by also preventing infections, and whether virus transmission is also interrupted.
Phase 3 clinical trial results have already told us about a vaccine’s likely impact on symptomatic infections. Clinical trials are important in providing the vital information on preventing symptomatic disease and determining safety and how the vaccine will work in certain target groups. However, it will only be after significant numbers of people have been vaccinated that we will have more complete information on extra benefits such as preventing hospitalisation, severe disease and reducing transmission.
As with all new medicines, we will monitor the vaccine’s effectiveness and safety to ensure we track and understand if any exceptionally rare side effects exist. As with all vaccination programmes, exceptionally rare side effects may not be apparent until millions of people worldwide have received the vaccine.
For the first phase of deployment, the UK and most other countries internationally are prioritising the available vaccine where it can be used to save lives and reduce hospital admissions of those most at risk from COVID-19. Such an approach is also the fastest way for a vaccine to support the lifting of non-pharmaceutical interventions (NPIs).
Places
Each of the UK nations has plans to scale up vaccine delivery and the following sections of this plan set out the deployment plans of the NHS in England. While exact models will differ slightly from region to region, all 4 nations’ plans involve a mixture of delivery models which include mobile teams to visit care homes, large sites at hospitals, and primary care-based delivery.
The COVID-19 vaccination is the biggest vaccination programme in NHS history. As of 7 January, the UK had vaccinated more people than the rest of Europe combined, and the arrival of the UK’s own Oxford/Astra Zeneca accelerated the pace of vaccination. By 15 February, we will have offered the first vaccine dose to everyone in the top 4 priority groups identified by the JCVI. That means offering the vaccine to all residents in a care home for older adults and their carers, everyone over the age of 70, all frontline health and social care workers, and everyone who is clinically extremely vulnerable.
The comprehensive planning considerations to date have included the size and make-up of the workforce, training requirements, guidance, consumables and other equipment, as well as the supporting infrastructure required, including warehousing, transport, logistics and ‘clinic’ storage. These plans build on the NHS’s tried and tested approach for delivering vaccinations across the country, including the annual influenza vaccine where we have this year already reached over 80% of over 65s. Our plans factor in:
-
who should offer a vaccination
-
geographical variation and diverse communities
-
access to public and private transport
-
ensuring NHS services are safe and accessible for people throughout the busy winter period
Safe, convenient and equitable access
The number of vaccination sites across the country will match expected vaccine supply. The capacity and mix of sites must also ensure safe, convenient and equitable access to vaccination in the order of JCVI cohort prioritisation. This requires the right clinical protocols, invitation and booking systems and clear public communication.
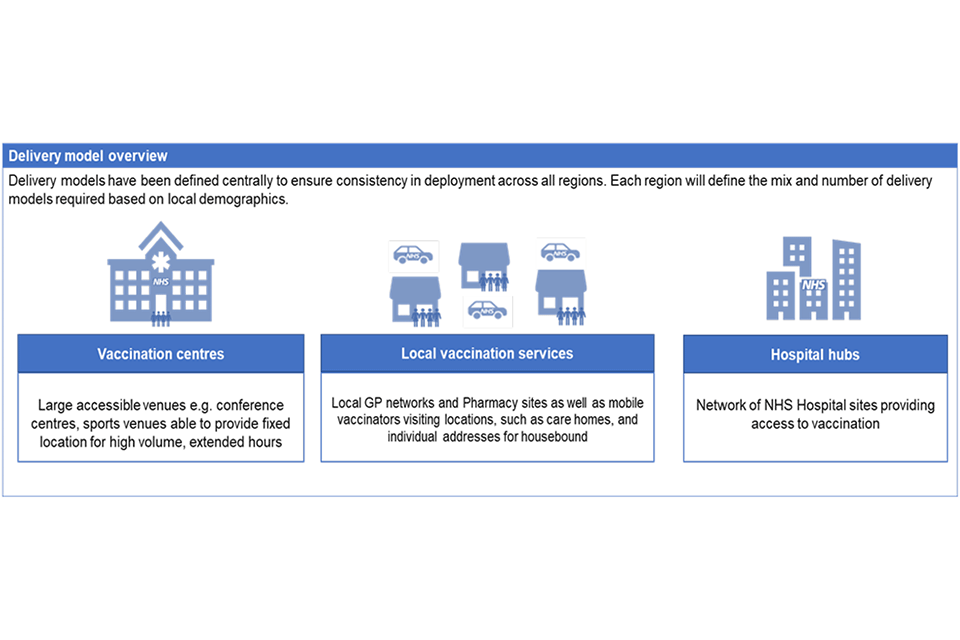
Vaccinations will be offered at:
Larger vaccination centres
A new approach in the NHS, these are large-scale venues, with higher throughput, using re-purposed venues, including sports stadiums, theatres, and hotels, located within communities to vaccinate large numbers of people. People will be offered an invitation and can book a slot that suits them using the National Booking Service.
Hospital hubs
These are based at NHS trusts, including acute, community mental health and ambulance trusts. They are targeting our health and care workers and will work closely with local authorities, local resilience forums and providers to coordinate rapid vaccination of the workforce. They are also excellent locations for initial deployment of new vaccines, so that all clinical safety issues can be identified and managed before wider roll-out. This has been the approach taken with the launch of the Pfizer/BioNTech and the Oxford/AstraZeneca vaccines.
Local vaccination services
These mobilise general practice, working together in groups of primary care networks plus large and small community pharmacy sites. These services provide the largest number of locations and are well placed to support our highest risk individuals, many of whom already have a trusted relationship with their local health services. They also coordinate and deliver vaccination to people who are unable to attend a vaccination site, including visiting care homes, the homes of housebound individuals and other settings such as residential facilities for people with learning disabilities or autism and prisons and to reach vulnerable groups such as those who are experiencing homelessness.
The plans for the right mix of vaccination sites have been developed jointly between national, regional and local teams to ensure the mix is right for the population and communities it serves. The needs of rural and urban communities will be very different, and the needs of individual groups and communities need to be reflected in the local mix of sites.
The mixed model will ensure that different communities access the vaccine in a way that is clinically and operationally secure. For example, it is not generally clinically appropriate for care home residents to go to hospital hubs. But others not resident in a care home are more able to receive the vaccination in a local vaccination service run by the general practice team that knows them best. Community pharmacy sites will start to deliver vaccines from mid-January, offering bookings through the National Booking Service. This will help improve access through primary care to as many of the population as possible. The early community pharmacy sites will be able to offer significant numbers of appointments. Community pharmacies are integral parts of local communities and will be accessible and approachable places from which to deliver vaccination.
All models have the required PPE and social distancing space to ensure that they are COVID-secure.
The different vaccine types will be used across the delivery models, although individual sites will typically only deliver one vaccine type per day. This approach is convenient for those receiving their vaccine, flexible to make best use of supply and maximise use of all our vaccine site capacity. There will be enough supply of the relevant vaccine type made available to make sure individuals can receive their second dose of the same vaccine type as their first dose.
Figure 4: overview of delivery models
The growing network of vaccination sites will rapidly expand in the weeks and months ahead. By late January we aim to have the capacity to vaccinate at least 2 million people each week. This will be achieved by increasing the number of hospital hubs, opening more vaccination centres, expanding local vaccination services and boosting primary care networks with community pharmacy sites. We will expand the programme so all adults can be vaccinated by the autumn.
In England, by the end of January, our capacity to vaccinate several hundred thousand a day, and at least 2 million people per week will be achieved by establishing:
-
206 active hospital hub sites
-
Around 1,200 local vaccination service sites (including primary care networks and community pharmacy sites)
-
50 vaccination centres
We are grateful for the offers from businesses up and down the country, including supermarkets and sporting arenas, to use their venues as vaccination centres. We have been working since the early summer to identify partners and work with those whose facilities have been identified as being suitable to provide support of the vaccine programme. At the moment the rate-limiting factor is not the number of vaccination locations, but we are ensuring that all offers are thoroughly considered, alongside local leaders, to understand potential partnerships that build on the existing network. We will also consider whether offers would be better suited for other areas of the response to COVID-19. To streamline this process, offers should be sent to vtf.support.offers@beis.gov.uk
Many partnerships are already in place. For example, Lord’s Cricket Ground recently opened as a local vaccination service and sites are also being used as large vaccination centres. One large vaccination centre will open in each of the 7 NHS regions this week with many more expected to be up and running by the end of January. The centres offer a convenient alternative to GP and hospital services and can each deliver thousands of vaccinations every week.
The first 7 sites are:
-
Ashton Gate in Bristol (South West)
-
Epsom racecourse in Surrey (South East)
-
Excel Centre in London (London)
-
The Centre for Life (North East and Yorkshire)
-
Etihad Tennis Club in Manchester (North West)
-
Robertson House in Stevenage (East of England)
-
Millennium Point in Birmingham (Midlands)
The initial sites were chosen from those ready to vaccinate large numbers of people quickly to give a geographical spread covering as many people as possible. As the map below shows, currently, 96% of the population in England is within 10 miles of a vaccine service. By the end of January, everyone will live within 10 miles of a vaccination centre. In a small number of highly rural areas, the vaccination centre will be a mobile unit.
Vaccine coverage will be reviewed and increased with support from our military advisors who compare provision against key data such as population density. The mobile model (where ‘roving’ vaccination teams bring the vaccine directly to individuals) which is being used to support the vaccination of care home residents and workers could be extended to more groups in time such as those experiencing homelessness, those escaping abuse in refuges, or communities with lower vaccination rates. Mobile models will also take the vaccine to those in the detained estate. The mobile model will also help more remote rural communities, particularly those at risk of isolation where public transport is limited.
Figure 5: map of vaccination sites as of 10 January 2021 (see a full list of sites on the NHS website)

Drawing on the military’s expertise
The UK Armed Forces are familiar with complexity and building things at pace. Plans need to be agile and the military’s tried and tested operational techniques include three key steps which we are applying to our vaccines target:
- analyse – assessing our site coverage against population density and identifying any gaps
Major Button said:
I am employed within the 101st Log Brigade and the SO2 Engineer. My role includes providing Military Engineer and Engineer Logistic advice to the Brigade Command. As part of my role as an engineer, I am often called on to analyse how the ground can impact on the delivery of a task. Throughout this task both electronic and physical mapping has been used to provide an accurate representation of the ground and the areas of operation. This allowed the team to identify any areas that were not within a 10-mile radius of a vaccine site.
- choose – identifying options for site expansion to address gaps
Major McGarry said:
As a Nursing Officer, I am fortunate to understand both clinical decision making and critical thinking, as well as how the military approach mission analysis, and military planning. Having a differing approach can be difficult to navigate when you have two differing organisations. I am able to think and converse in both NHS and military manners. Being both an Army Officer and Nurse helps with other fellow health care professions, and my military colleges having both a shared commonality and knowledge base, and where required translating one into the other.
- execute – delivering an expansion plan at pace with command and control oversight of activity on the ground
Lieutenant Lambert said:
I am a Submariner, with a specialty as a Logistics Sea Trainer. As advisor to the vaccination operation, the execute phase has seen engagement with various stakeholders to enable information flow, understanding and early identification of emerging issues. This has included helping design an operational view of the programme by identifying key performance indicators to aid us in spotting potential problems and providing solutions in a timely fashion.
The online National Booking Service identifies individuals based on age and will be used to invite and book people in for their vaccination at a large vaccination centre. You can use this service if you receive a letter inviting you to book your vaccination appointment. If you are unable to use the internet, someone else is able to use the service on your behalf or you can call our helpline by phoning 119 free of charge. On the helpline you can talk to someone who can support you to make your booking. The helpline is also designed to provide assistance and to signpost to further information. If required, the helpline can provide support in different languages.
Employers are best placed to help us in identifying health and social care workers and are working closely with hospital hubs. A system to invite and validate eligibility of health and social care workers rapidly onsite is being launched. Building on their connections to their community, especially those with long term conditions, primary care will play a crucial role in identifying and inviting those who are clinically extremely vulnerable. People may receive multiple invitations and have a choice of sites from which to decide what suits them best. At the start of February, data from the National Booking Service will be combined and cross-referenced with GP records to identify any individuals who have not been invited.
In partnership with the Home Office and DCMS, we are working to protect the public from fraudulent communications and misinformation from criminals who seek to exploit individuals by asking people to pay for ‘vaccines’ or using fraudulent invitation systems to access people’s personal data. All vaccines are free, and the NHS will never ask you for bank details or payment information.
How you will be contacted and what happens next
Figure 6: vaccination user journey
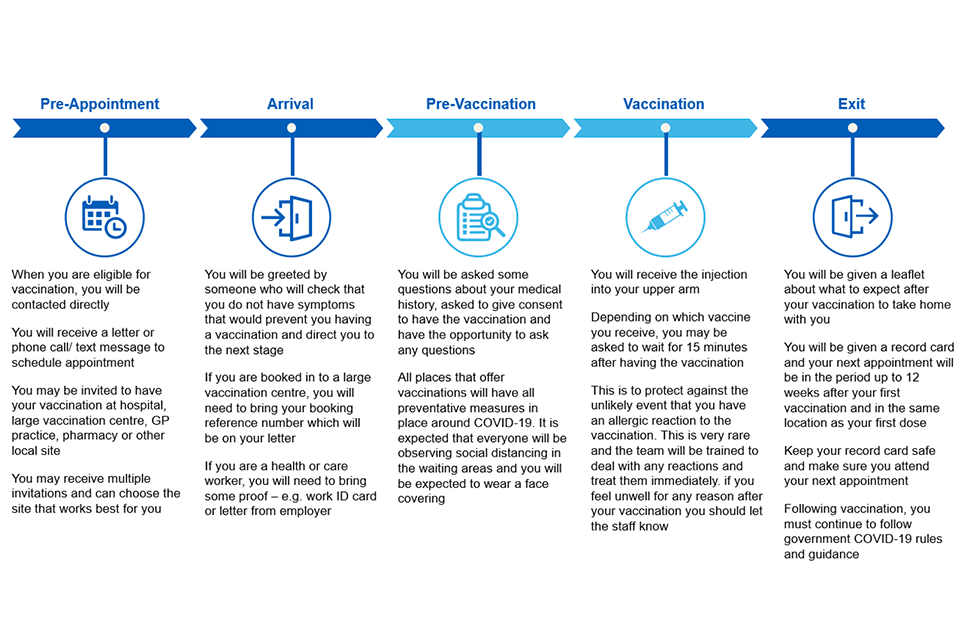
The NHS outlines the information contained in this image.
Bringing the vaccine to care home residents and care staff
There is clear evidence that those living in residential care homes for older adults have been disproportionately affected by COVID-19 as they have had a high risk of exposure to infection and are at higher clinical risk of severe disease and mortality. Given the increased risk of outbreaks, morbidity and mortality in these closed settings, these adults are considered to be at very high risk. The JCVI’s advice is that this group should be the highest priority for vaccination. Vaccination of residents and staff at the same time is considered to be a highly efficient strategy.
Since December, we have been taking vaccines directly to care homes. This is a joint effort between the care homes and the primary care network vaccinating teams. Not only has this provided protection from COVID-19, the vaccine has brought a huge lift in mental wellbeing for care home staff, residents and their relatives. Recognising that care home managers know their residents best, local vaccinating teams are working closely with care home managers. This has supported the consent process and helped with understanding practicalities (for example care home layouts; agreeing where to vaccinate and observe residents; and determining how to maintain the cold chain transport requirements of the Pfizer/BioNTech vaccine). Packing down the vaccine into allocations of 75 doses has enabled efficient use in large care homes with over 50 beds.
It is our ambition to offer the vaccine to all care home residents and staff in the more than 10,000 care homes in England for older people by the end of January. This has been greatly helped by the authorisation of the Oxford/Astra Zeneca vaccine. The vaccine can be stored at fridge temperatures, between 2 and 8 degrees, making it easier to distribute to care homes. Deliveries of both vaccines to large (over 50 beds), medium (25 to 49 beds) and small (under 25 beds) care homes are underway.
Social care staff work in a range of settings beyond care homes, not least in domiciliary care, which makes up a significant proportion of the sector. The diversity of the sector and its workforce has put a premium on strong local leadership and partnerships between local authorities, the NHS, social care providers and their representative bodies. Close joint working between these partners, with local authorities playing a lead role, will be essential to identifying and offering a vaccine to all eligible frontline social care workers. To give just one example, it is vital that we identify and reach directly employed personal assistants, who support people within any of the JCVI priority groups (the clinically vulnerable and adults) as part of our efforts to ensure full coverage of the priority 2 cohort.
Understanding our communities and providing them with the advice and information they need to take up the vaccine
The Department of Health and Social Care, NHS, PHE and local authorities are providing advice and information at every possible opportunity to support those who have been prioritised to receive a vaccine and anyone who has questions about COVID-19 vaccines and the vaccination process.
Working with all communities to provide advice and information about vaccines is central to ensuring as many people as possible take up the offer of a COVID-19 vaccination when it is their turn. The NHS website includes information on how you will be invited and what happens next.
We know that the vast majority of people want to receive a vaccine but we need to ensure that our vaccination programme is inclusive and helps tackle inequalities by addressing individual concerns of those who have questions about how the vaccine fits their particular circumstances such as their age, ethnic background or a medical condition.
At national, regional and local level we will be working in partnership with local authorities, the voluntary and community sector, local resilience forums, communities, staff and patients to ensure that simple accessible advice and information is available to everyone who needs it.
Working together with partners, we are taking a strategic approach that aims to support individuals, especially those at risk of exclusion, with improved access to information and services. We will ensure all material is available in a range of formats, including translations, easy read, braille and accessible for those with hearing impairment.
At a national level, an Equalities Board has been established to ensure our approach has health inclusion at its heart. We also know that black, Asian and minority ethnic (BAME) communities have been disproportionately impacted by COVID-19, and this extends to our NHS health and care workforce. To address this head on, a dedicated team to support effective communication with BAME staff, headed by Dr Nikki Kanani, NHSEI Medical Director of Primary Care and NHS Chief People Officer, Prerana Issar, has been established. Recognising our workforce are our biggest advocates and leaders within their own communities, this ensures all staff communications are relevant, accessible and specific and the view and priorities of BAME staff are part of the conversation.
Drawing on local authorities’ knowledge of their communities
Local authorities, working with local NHS colleagues, know their communities best and will play a key role in supporting this, through the expertise of directors of public health and their teams and their wider skills to address diversity and community development. We are committed to ensuring that local authorities and directors of public health have the data they need to understand uptake in their local areas and tailor efforts to reach those who have not yet taken up the vaccine. Local authorities have already been researching the impact of COVID-19 within their communities and we will work closely with them to provide the information they need about COVID-19 vaccines and the vaccine programme to engage actively with communities in the way they feel will work most effectively.
Local authorities leading the way
A number of local authorities are already proactively working in this area, with Hertfordshire County Council leading significant research in this area, published in December 2020 and sharing more widely within the sector. This focuses on 3 Cs:
- finding ways to reduce complacency regarding the risks of COVID-19
- building confidence in the safety and effectiveness of the vaccine
- increasing the convenience of being vaccinated
The national programme is supporting the sharing of this approach and other best practice examples emerging with further webinars hosted to the Local Government Association planned in 2021 for communications and behavioural insight and research teams.
MHCLG is funding the Community Champions Programme which will work with up to 65 local authorities across England to boost work to reach out to ethnic minority and disabled communities. This will include intensive engagement by community voices around vaccinations – learning and other resources from local activity will be shared to a wider audience.
Meaningful community engagement is also being led by local Integrated Care Systems (ICSs) across the country. For example, Devon ICS are working with national advocacy organisation Friend, Families amd Travellers to produce a best practice guide to engaging with the traveller community which will include community informed communications and be shared across the country. Building on lessons learnt, Bristol, North Somerset and South Gloucestershire ICS are developing best practice approaches to engaging and supporting people experiencing homelessness, including providing accessible information and supporting GP registration. This is supported and shared nationally through well-established networks of frontline outreach workers and practitioners. These are just 2 examples of community engagement that is going on up and down the country; systems are working hard to engage effectively with their local communities, through established community leaders, such as faith leaders, or by working with local partners to address the concerns and meet the needs of communities.
People
Mobilising the workforce
The NHS has a strong history in primary and community care delivery of vaccination programs and the COVID-19 programme will build on this to ensure the safe and effective delivery of vaccination to people across the country. Local NHS leaders are working with partners across the NHS, local authorities, health and social care partnerships and voluntary sector to build up the workforce.
At its heart is the NHS staff in hospitals, community and primary care. We have trained an 80,000 strong vaccination workforce. Staff have been identified to meet supply in all three delivery models. NHS staff continue to fight the pandemic, taking on additional COVID-related activities as well as continuing to maintain services for other healthcare needs. The national vaccination effort has also been boosted by many former clinicians, care staff, and students.
New workforce legislation means a wider group of healthcare professionals are able to safely administer the COVID-19 vaccine and this will improve access to vaccines while minimising any impact on existing services. This includes drawing on the skills of those who have volunteered through the NHS Bring Back scheme, considering the use of a wider range of professionals such as registered dentists, midwives, occupational therapists, paramedics, physiotherapists and radiographers, trainee doctors and nurses, as well as those currently working outside of the NHS such as St John’s Ambulance and independent nurses and occupational health service providers.
This expanded workforce will work alongside GPs, nurses, pharmacists and other professionals in primary, community and acute care experienced in delivering vaccination programmes. There are many other people in the UK who have experience and vital skills that can be adapted to support vaccination services. For example, cabin crew are being recruited and trained to work alongside NHS colleagues at vaccination sites.
As well as trained vaccinators, the COVID-19 vaccination deployment programme will include a range of non-clinical support staff to ensure people have quick and easy access to vaccination sites. For example, administration support, logistics, stewards and first aiders, as well as those who can log, record and manage stocks are all crucial to the effort. Additional resource will be made available to primary care to support primary care teams through the national contract, and larger sites will be staffed by volunteers from St John’s Ambulance for example, who can supply vaccinators and thousands of non-clinical volunteers to help. The armed forces have been supporting the vaccination program with logistical expertise, leadership and advice.
Volunteers, including NHS Responders, coordinated by the Royal Voluntary Service, will play a crucial role as stewards helping people throughout their visits to vaccination centres.
“Five bays giving out the vaccine and in virtually constant use. So well organised there was a constant throughput but no queuing. Clerks in every bay doing the computer records bit freeing up the nurses to ask the relevant questions and give the vaccine”
Communicare in Southampton is a charity with over 300 volunteers who provide physical and emotional support to lonely and isolated people. They are working in partnership with GP surgeries in Southampton and Solent NHS Trust, a lead provider in Hampshire and the Isle of Wight for COVID-19 vaccination services, to support the rollout of the vaccination. The charity has received lots of interest from people signing up to volunteer.
Volunteers have been:
- marshalling in the car park
- helping people out of cars and to their appointments
- sitting with people in the reception and recovery areas, providing chat and neighbourly support
- reminding people of the importance of ‘Hands. Face. Space’
More than 200,000 people have expressed an interest in joining the vaccination programme. Not everyone who has volunteered will be deployed straight away, but we will ensure that people are kept informed, so they can be mobilised if needed in the months ahead to ensure we always have the workforce to meet vaccine supply. Where we are oversubscribed in some areas, we may ask that volunteers help the NHS in other ways this winter.
