Regulator warns against purchasing inaccurate infrared thermometers
The public and healthcare professionals are being warned to take care when purchasing infrared thermometers online and to familiarise themselves with warning signs for poor quality products.
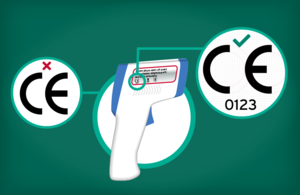
- The Medicines and Healthcare products Regulatory Agency (MHRA) is warning the public and healthcare professionals to take care when buying infrared thermometers and to know what to look for when purchasing online
- There has been an increase in the number of unreliable infrared thermometers which are available in the UK
- Infrared thermometers, like all medical devices fit for sale on the UK market, will carry a distinct CE or UKCA mark
The MHRA has seen an increase rise in the number of inaccurate infrared thermometers making their way onto the UK market –this is believed to be caused by the COVID-19 pandemic creating a massive increase in demand for these products.
Contactless infrared thermometers are widely used to check people’s temperature as a method for screening for a raised temperature, and possible COVID-19 infection. Products marketed with claims that they can be used for a medical purpose are medical devices and therefore regulated by the MHRA.
Graeme Tunbridge, Director of MHRA Devices division said:
People in the UK are unknowingly buying poor quality infrared thermometers which do not meet the required standards of safety and performance.
It is important that people know what to look out for when purchasing these products, or any other medical devices online. You should always look for the CE or UKCA mark on any medical device – this will either be on the device or on its packaging, and that includes when you are buying these products online.
The MHRA advises that anyone considering purchasing an infrared thermometer should follows the guidance set out in the information for the public and professional users here. People can also read our general advice and tips on purchasing medical devices online here.
In addition, people should be aware that temperature screening is not a reliable method for detecting SARS-CoV-2 infection. In July 2020, the MHRA issued a warning to manufacturers and suppliers of thermal cameras which cautioned them against making claims which directly relate to COVID-19 diagnosis.
The MHRA is responsible for enforcing the law on medical devices in the UK and has a range of a range of investigatory and enforcement powers to ensure their safety and quality. If suppliers fail to comply with the regulations then they may be subject to prosecution.
Notes to editor
- The Medicines and Healthcare products Regulatory Agency is responsible for protecting and improving the health of millions of people every day through the effective regulation of all medicines and medical devices in the UK by ensuring they work and are acceptably safe. All our work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks.
- The MHRA is a centre of the Medicines and Healthcare products Regulatory Agency which also includes the National Institute for Biological Standards and Control (NIBSC) and the Clinical Practice Research Datalink (CPRD). MHRA is an executive agency of the Department of Health and Social Care.
- Further detailed information on medical device regulation in the UK and CE / UKCA mark can be found on this page
- For general advice and tips on purchasing medical products visit our web pages for buying medical devices and buying medical products online..
Media enquiries
News centre
MHRA
10 South Colonnade
London
E14 4PU
Email newscentre@mhra.gov.uk
During office hours: 020 3080 7651 (08:30 - 17:00)
Out of office hours: 07770 446 189 (17:00 - 08:30)
Office hours are Monday to Friday, 8:30am to 5pm. For real-time updates including the latest press releases and news statements, see our Twitter channel at https://www.twitter.com/mhragovuk.