Company led medicines recall: Prulab Pharma Limited, Clopidogrel Oral Solution 75mg in 5ml [unlicensed medicine], CLMR (23)A/02
Prulab Pharma Limited is recalling a batch of Cloipidogrel Oral Solution 75mg in 5ml due to a discrepancy with the product labelling that could result in an incorrect volume to be administered to the patient.
CLMR Number
CLMR (23)A/02
MDR Number
MDR 054-01/23
Company name
Prulab Pharma Limited
Product description
Clopidogrel Oral Solution 75mg in 5ml [unlicensed medicine]
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| D2207227403B | 21 July 2023 | 150ml | 05 September 2022 |
Active ingredient: Clopidogrel hydrogen sulfate
Brief Description of Problem
Prulab Pharma Limited is recalling the above batch of Clopidogrel Oral Solution 75mg in 5ml due to a discrepancy with the product labelling that could result in an incorrect volume to be administered to the patient.
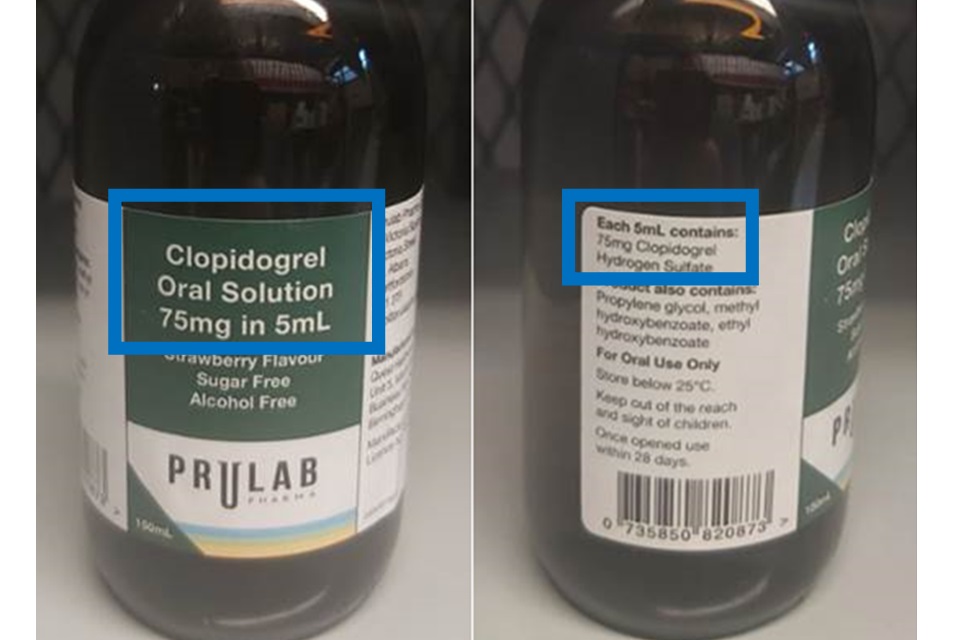
The strength of the formulation reflected in the product name on the front label does not align with the quantitative composition of the product, which states “Each 5ml contains 75mg clopidogrel hydrogen sulfate”.
Clopidogrel doses are generally expressed in terms of the base: 75mg of clopidogrel is equivalent to 97.86mg of clopidogrel hydrogen sulfate. For this product, each 5ml contains 75mg clopidogrel hydrogen sulfate, which is equivalent to 57.48mg of clopidogrel. Without taking into account the equivalence of the active pharmaceutical ingredient in this product, a patient may be receiving an underdose of this medicine.
This recall is being issued as a company-led medicines recall, as this product is only supplied to small number of customers, and the manufacturer has full traceability of the product’s distribution.
Remaining stock of the above batch should be quarantined and returned to the company directly.
Advice for healthcare professionals
Stop supplying the above batch immediately. Quarantine all remaining stock and return it to your supplier using your supplier’s approved process.
Due to the discrepancy in the labelling of this product, there is a risk that the calculation for the volume of medicine to be administered may not have been appropriate.
Pharmacists who have dispensed this unlicensed medicine should review all prescriptions to ensure that the volume of administration corresponds to the clopidogrel dose prescribed. Further instructions are provided in the Direct Health Professional Communication (DHPC).
Advice for patients
Patients and caregivers who have been dispensed with this unlicensed medicine will be contacted by their clinical teams to confirm that the volume of medicine they are taking is appropriate for the dose prescribed for them. Patients should not change their dose of oral clopidogrel unless they are told to do so by a healthcare professional.
Patients should note that there are various preparations of clopidogrel oral solution. The dosing volume is specific to each formulation and may not be the same for different brands.
Any suspected defective medicine should be reported via the MHRA Yellow Card scheme.
Patients who experience adverse reactions or have any questions about the medication, should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Company Contact for medical information enquiries
Jackie Peck, Pharmacy Consulting Ltd: jackie@pharmacyconsulting.co.uk
Telephone: 01252 375362 / 07909 836670
Company Contact for stock enquiries
Andy Oades, Director: andy@prulabpharma.co.uk
Telephone: 07921409899
Download document
Direct Healthcare Professional Letter - Prulab Pharma Limited, Clopidogrel Oral Solution 75mg in 5ml